|
Pharmacogn Rev. 2022;16(31):34-39 A multifaceted peer reviewed journal in the field of Pharmacognosy and Natural Products www.phcogrev.com | www.phcog.net |
Review Article |
Reactualiting Phanera semibifida (Roxb.) Benth. from Sumatra, Indonesia as an Anticancer Agent and Phylogenetic Position
Fitmawati1,*, Erwina Juliantari2, Rodesia Mustika Roza1, Titrawani1, Vanda Julita Yahya1
Fitmawati1,*, Erwina Juliantari2, Rodesia Mustika Roza1, Titrawani1, Vanda Julita Yahya1
1Department of Biology, Faculty of Mathematics and Natural Sciences, Universitas Riau, Pekanbaru, Riau, INDONESIA.
2Plant Biology Graduate Program, Department of Biology, Faculty of Mathematic and Natural, IPB University, Bogor, West Java, INDONESIA.
Correspondence
Prof. Dr. Fitmawati, M.Si
Department of Biology, Faculty of Mathematics and Natural Sciences, Universitas Riau, Pekanbaru, Riau, 28293, INDONESIA.
E-mail: [email protected]
History
• Submission Date: 26-11-2021;
• Review completed: 15-12-2021;
• Accepted Date: 20-01-2022.
DOI : 10.5530/phrev.2022.16.6
Article Available online
http://www.phcogrev.com/v16/i31
Copyright
© 2022 Phcog.Net. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International license.

ABSTRACT
The increasing prevalence of cancer worldwide demands the intensification of efforts to develop natural solutions for its treatment. Herbal plants have provided a strong evident base in treating of this disease in many indigenous tribes in the world. Phanera semibifida has been widely used as a viable anti-degenerative drug for cancer treatment. This study reviews selected plant species with taxonomy status, phylogeny position, phytochemistry and pharmacology study based on a author’s research and literature review conducted through relevant keyword searches in databases, Web of Science, Scopus, PubMed, and Google Scholar. Detailed analysis of research studies proves that P. semibfida can be used as an anticancer agent with strong basic evidence based on indigenous knowledge from traditional communities.
Key words: Anticancer, Leguminosae, Phanera semibifida, Pharmacology, Sumatra.
Cite this article: Fitmawati, Juliantari E, Roza RM, Titrawani, Yahya VJ. Reactualiting Phanera semibifida (Roxb.) Benth. from Sumatra, Indonesia as an Anticancer Agent and Phylogenetic Position. Pharmacog Rev. 2022;16(31):34-9.
INTRODUCTION
In the human body, free radicals are considered to play a role in the process of disease. The imbalance between the content of free radicals and antioxidants in the body becomes the dominant cause of free radicals, resulting in various diseases such as cancer, coronary heart disease, diabetes, liver and premature aging.[1] Antioxidants are compounds that can prevent the occurrence of oxidation reactions of other compounds both in the body and for other compounds that are easily oxidized.[2] Antioxidants are chemical compounds that can donate one or more electrons to free radicals, so that free radical can be reduced by their radical properties.[3] Currently, exposure to free radicals is quite widespread in people’s lives, such as pollution and lifestyle.[4] Plants containing antioxidant compounds have an important role in increasing immunity in the body.[5-8]
The immune system is the body’s defense mechanism in charge of responding or responding to attacks from outside the body, such as bacteria, viruses, fungi, and various germs that cause disease.[9,10] When the immune system does not work optimally, the body will be vulnerable to infection. Several things can affect the immune system, such as environment, food, lifestyle, stress, age, and hormones.[11,12] Immunomodulator serves to develop materials that can enhance the immune response or restore the immune systems’s.[13,14] Improving the immune system can be done in various ways, one of which is by consuming of supplements that function as immunomodulators.[15-17]
Various diseases continue to undergo massive differentiation and quickly attack many people due to lifestyle changes. The lifestyle of consuming fast food and lack of activity can increase the risk of heart disease, stroke, diabetes, and cancer, which are better known as degenerative diseases. Based on WHO data degenerative diseases are the main cause of death globally with around 54%. The increasing prevalence of degenerative diseases such as cancer requires scientists to find new sources of natural ingredients as potential natural antioxidants.[18-20]
Globally cancer is a disease that greatly affects the human population. Many scientific studies to find potential compounds from herbal plants as targets for potential anticancer treatments.[21] Plants produce natural secondary metabolites that are being investigated for anticancer activity leading to new clinical drugs.[22] Natural herbs have harmless side effects compared to current treatments such as chemotherapy.[23]
Kangkang katup (Phanera semibifida (Roxb.) Benth.) has strong basic evidence in traditional Malay medicine in the Riau Islands, Sumatra, Indonesia. Previous studies have revealed that valve ribs are a source of antioxidants and immunomodulators.[24,25] This evidence makes a major contribution to revealing the potential of natural resources as anticancer drugs. This review will explore the chemical diversity of P. semibifida which has potential as an anticancer with clear taxonomic and phylogenetic identity authentication.
TAXONOMY OF PHANERA SEMIBIFIDA
Naming is the key to unlocking all information about an organism. If the name is not clear. The information about the organism is also invalid and not recognized by the scientific community. Kangkang katup (Phanera semibifida) is a herbal plant always present in every traditional medicinal herb, such as in the Riau Lingga Malay tribe, Riau Islands Province. A valid name is very important in developing the potential of plants as medicinal plants. Phanera semibifida (Roxb.) Benth is an accepted name by The International Plant Names Index (IPNI), basionym with Bauhinia semibifida Roxb. from Fabaceae (leguminosae: Caesalpinioideae). Originally Phanera was a subgenus of Bauhinia. However, it has been revised by de Wit,[26] as the most recent genus in Bauhinia with specialization characters is nearly entirely composed of climbers and the presence of three fertile stamens.
Phanera semibifida (Roxb.) Benth. in Miquel, Pl. Jungh. (1852) 263; Miquel, Fl. Ind. Bat. 1 (1855) 61; de Wit, Reinwardtia 3 (1956) 465 (Figure 1).[26-28]
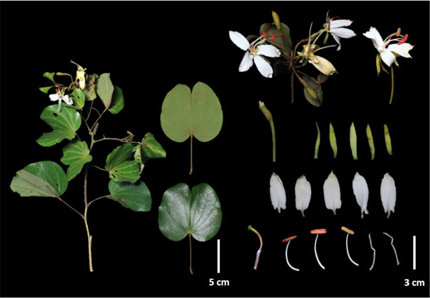
Figure 1: Morphological characters of Phanera semibifida grown in West Sumatra, Sumatra.
Perennial, woody climber. Leaves simple, butterfly-shaped (with bifoliolate consisted of two leaflets), green and glabrous above. Petiole 2-6 cm, pubescent to glabrous. Lamina obovate-orbicular, 4-11 cm diam; (9-)11-13-nerved; bifid 1/4 - 2/5, tips of lobes rounded, base cordate, entire, curved; upper surface dark green, glabrous, lower light green, glabrous to glabrescent. Inflorescences start out white and age to yellow, fragrant, terminal, elongated erect racemes. Flower buds mature from fairly glabrous white to pubescent golden brown, opening sequentially from bottom to top. Calyx early splitting into 5 reflexed, strap-shaped sepals, 1-2.5 cm long. Petals white turning yellow, unequal, elliptic to oblong, glabrous except for the pubescent claw, and sometimes sparsely hirsute externally along the median line, 2-3(-3.5) cm long, claw 2-5 mm. Stamens 3 fertile; filaments 2.5-3 cm, glabrous, stout; anthers oblong, 1 cm, opening by a longitudinal slit; staminodes 2-3, small, filiform. Stipe of ovary c. 3 mm, ovary c. 10 mm, style stout, c. 5 mm, all entirely densely brown silky tomentose; style increasing towards the very large oblique, peltate stigma, up to 5 mm across, much lengthening after anthesis. Fruits like dried, woody, strap-straped, bean pods 10–20 by 3–4 cm. Each fruit bears up to 6 disc-like, flat seeds within.
Distribution: P. semibifida is a tropical plant with native distribution in Peninsular Malaysia, Myanmar, the Philippines, Borneo Sulawesi, and Sumatra.
Habitat: It grows along with open areas in forests, and forest edges up to 2000 m altitude
Vernacular names: Ganggang katup (Lingga); Kupu-kupu (Borneo, Pontianak); Takui lebang (Sungei); Akar kati katwi (Sumatra, Mt Ophir), Andor sibola ringring (Asahan), Bulun siduaja (Simalur), Akar pulalang (Jambi).
MOLECULAR PHYLOGENY ANALYSIS OF PHANERA SEMIBIFIDA
We collected two accessions of Phanera semibifida from Sumatra: Riau Islands and West Sumatra. The relatively closely related species was selected as the outgroup for phylogenetic analyses based on ITS sequences.[29] DNA sequences of ITS region of species and outgroup taxa were aligned by Unipro UGENE.[30] Maximum parsimony analysis was performed using PAUP*.[31] Clade robustness was evaluated by bootstrap analysis using 1000 replicates of heuristic searches.[32]
The result of BLAST indicated that two accession P. semibifida has a high similarity to P. semibifida (AY258395.1) with identity value ≥ 95% while the similarity to both P. nervosa (HG004798.1) and Bauhinia championii (KP092701.1) is less than 95% (Table 1). Using BLAST parameters are important to determine species name.[33]
Table 1: BLAST analysis of ITS region sequences of species from Riau Islands and West Sumatra.
| Description | Species | Max score | Total score | Query cover (%) | E value | Ident (%) | Accesccion |
|---|---|---|---|---|---|---|---|
| Bauhinia semibifida internal transcribed spacer 1, 5.8S ribosomal RNA gene, and internal transcribed spacer 2, complete sequence (common name: Phanera semibifia). | P. semibifida_1 | 1101 | 1101 | 87 | 0.0 | 99.02 | AY258395.1 |
| P. semibifida_2 | 937 | 937 | 96 | 0.0 | 95.02 | ||
| Lasiobema championii 18S ribosomal RNA gene, partial sequence; internal transcribed spacer 1, 5.8S ribosomal RNA gene, internal transcribed spacer 2, complete sequence; and 28S ribosomal RNA gene, partial sequence (common name: Bauhinia championii). | P. semibifida_1 | 926 | 926 | 98 | 0.0 | 90.90 | KP092701.1 |
| P. semibifida_2 | 800 | 800 | 96 | 0.0 | 88.05 | ||
| Bauhinia nervosa 18S rRNA gene, ITS1, 5.8S rRNA gene, ITS2 and 28S rRNA gene (common name: Phanera nervosa). | P. semibifida_1 | 918 | 918 | 98% | 0.0 | 90.61 | HG004798.1 |
| P. semibifida_2 | 880 | 880 | 96% | 0.0 | 90.25 |
P. semibifida_1 collected from Riau Islands, and P. semibifida_2 collected from West Sumatra.
The phylogenetic tree of P. semibifida based on Internal Transcribed Species (ITS) markers sequences showed kinship with the genus Phanera and its relatives. Phylogenetic analysis is based on a representative sample of the genera Phanera and Bauhinia, including nine accessions taken from GenBank data. The phylogeny tree reveals two main clades, supported by a high bootstrap value. Two accessions of P. semibifida formed separate clusters from other species (Figure 2). Differences from environmental factors in their habitat can change nucleotides the basic constitution of the species,[34] and the chemical components between species that grow in different countries could vary due to climate and another geographic factor.[6]
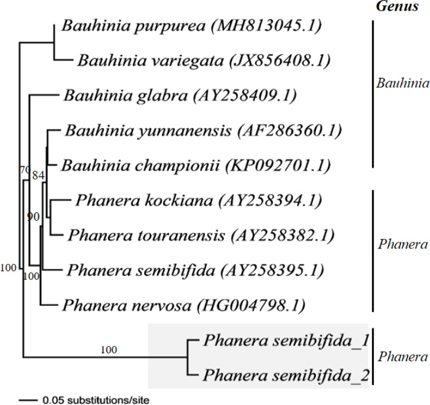
Figure 2: Cladogram of two species of Phanera semibifida as compared to related species based on ITS marker with the neighbour-joining method with bootstrap values 1000x. The numbers above lines represent bootstrap values in 1000 replicates. P. semibifida_1 collected from Riau Islands, and P. semibifida_2 collected from West Sumatra.
All of these segregate genera in Bauhinia based on trnL-F, and ITS region is monophyletic except for Phanera, which is divided into two lineages comprising the Asian Phanera species together with the genus Lasiobema, and the second containing the American Phanera species.[35-37] Phanera is probably better recognized as two lineages, one comprising neotropical Phanera, corresponding to the genus Schnella, and the other containing the Asian Phanera and Lasiobema species. However, more sampling and more variable molecular markers are needed to resolve the taxonomic status of Phanera and Lasiobema.[35]
Cladogram based on ITS region showed two accession P. semibifida from Sumatra identical to P. semibifida that grows in other countries. Based on the NJ analysis, the species collected from Sumatra were evolutionarily advanced because they had more extended branch sizes than other species collected from the GeneBank data. After all, they had more extended branch sizes. The genus Phanera differs from Bauhinia based on morphological and molecular characteristics. These findings are important in authenticating herbal plants for the development of pharmacological studies.
PHYTOCHEMISTRY OF PHANERA SEMIBIFIDA
The study about phytochemical of Phanera has known that these plants produce flavonoids,[24,38,39] and stilbenoids.[40-42] Phytochemical studies on one species of the genus Phanera, namely P. sembifida, two flavonoid compounds have been found that dominate. There were 6C-7O-dimethylaromadendrin (1), and phlorizin (2) (Figure 3) that have been isolated from the stem bark of P. semibifida. The flavonoid 4’,5,7-trihydroxy-6,8-dimethylflavanone (farresol) and 3’,4’,-4trihydroxy chalcone (isoliquiritigenin) together with triterpenoid (friedelin) were isolated from the aerial parts of P. bracteata.[43] In general, a positive relationship appears to exist between total flavonoid content and antioxidant activity.[44]
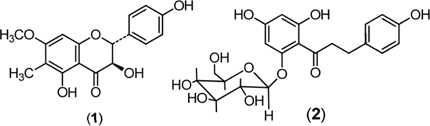
Figure 3: Structures of flavonoid isolated (1). 6C-7O-dimethylaromadendrin and (2) phlorizin.[47]
The difference in the flavonoid content of a plant is influenced by sunlight, an increase or decrease in sunlight affects the production of flavonoids. Enzyme activity in the formation of flavonoids in the photosynthesis process is highly dependent on sunlight. The higher the sunlight, the higher the transpiration rate. The photosynthesis results in carbohydrates are then processed in the cell to become flavonoid content in plants.[45,46]
Flavonoids are a group of phenolic compounds with antioxidant potential and play an important role in protecting against oxidative stress. Functional hydroxyl groups in flavonoids mediate their antioxidant effects by scavenging free radicals, and chelating metal ions.[48] Flavonoids are one of the important components in nutraceuticals, pharmaceuticals and medicines. This is due to its antioxidant, anti-inflammatory, anti-mutagenic, and anti-carcinogenic properties coupled with its capacity to modulate cellular keys enzyme function.[46]
PHARMACOLOGY OF OF PHANERA SEMIBIFIDA
Kangkang katup (Phanera semibifida) is one important component in Lingga Malay tribe herbal medicine,[24] because it has high antioxidants. This species not only grows on the islands but can also be found in Riau mainland. Previous studies have revealed that valve ribs are a source of antioxidants and immunomodulators.[2,25] This evidence significantly contributes to revealing the potential of natural resources as antidegenerative drugs due to changes in people’s lifestyles known in certain tribal communities and other areas.
Antioxidant Activity
Antioxidants are a group of chemical compounds that can suppress cell damage caused by free radicals by giving electrons speedily and transforming the free radicals into stable forms to prevent oxidative damage that causes ailments.[49] The presence of antioxidants in plants is primarily a protective compound from pests and disease, known as bioactive compounds. In this study, the quantitative phytochemical analysis was purposely conducted to determine the antioxidants and content of flavonoid and phenolic compounds, specifically gallic acid and quercetin. These analyses were performed using the DPPH method, and the concentration of gallic acid and quercetin were analyzed using HPLC.
As a traditional medicinal plant, all parts of P. sembifida have been tested for their antioxidant content, starting from the roots, stems, and leaves. Methanol extract obtained from maceration is rated to have an IC50 value of 16 g/Ml with an AAI value of 2.5, which is categorized as stronger than root and leaves (Table 2).[50]
Table 2: Antioxidant activity in the root, stem bark and leaves of P. sembifida.
| Organs | IC50 (ug/mL) Methanol | Antioxidant Activity Criteria* |
|---|---|---|
| Root | 6.6247 | Very strong |
| Stem bark | 16 | Very strong |
| Leaves | 6.3486 | Very strong |
Based on the IC50 value, the three P. sembifida plant organs can be used as medicinal herbs because they have high antioxidant activity. The community uses several genus Bauhinia and Phanera in traditional medicine, such as antitumor, bronchitis, dysentery, diabetes and antimicrobials.[51-56] The part of the Phanera plant used as a herbal concoction is generally in the form of a decoction of root bark, stems and leaves.
Immunomodulator activity
Phanera sembifida waas used by the traditional Malay community of Linga as a Obat Pahit herb. Every Traditional Medicine Practitioner (TMP) used this plant as a medicinal herb that was believed to be herbal medicine as a body energy keeper (immunomodulator and antioxidants).[24] When the immune system works unwell, our body will be easily susceptible to disease infections.
This immunomodulatory effectiveness study has explored two aspects: activity and capacity of phagocytosis. Phagocytosis cells are predominantly important in the removal of bacteria and parasites from the body. They engulf these foreign bodies and degrade them using their powerful enzymes.[57] Phagocytosis activity is the number of phagocyte cells ingesting antigents like bacteria, while phagocytosis capacity is the number of bacteria cells that are ingested by phagocytic cells.
P. sembifida extract showed an increase in phagocytosis against Staphylococcus aureus (SA) No. ATCC 12600 activity and phagocytic capacity to effectively modulate the immune system.[25] P. sembifida extract can be used as an immunomodulatory raw material traditional knowledge-based herbal medicine. It also has potential as a standardized herb.
Anticancer Activity
Anticancer activity 6C-7O-dimethylaromadendrin and phlorizin using MTT assay has been widely used to assess cell viability. However, one must consider the enzymatic reduction of 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT) to MTT-formazan is catalyzed by mitochondrial succinate dehydrogenase. Anticancer activity test against Murine leukemia P-388 cells showed IC50 values of 2.76±0.08 ppm and 6.16±3.50 ppm. The IC50 value obtained was included in the moderate and inactive activity categories. The cytotoxic activity of the ethyl acetate stem extract of P. semibifida obtained the IC50 value of 72.93±0.01 ppm.[58]
Anticancer activity assays were also proven in some relatives of P. semibifida, such as the flower of P. kockiana with gallic acid and methyl gallate that exhibited anticancer properties towards cancer cell lines.[59] The antitumour potential of Bauhinia variegata against B16F10 melanoma tumor model in C57BL mice. The present investigation was undertaken to explore the antitumor activity of leaf, stem bark and flower extract of Bauhinia variegata against B16F10 melanoma tumor model in C57BL mice.[60] The present study demonstrates the anticancer activity of extract Bauhinia tomentosa. The ethanol leaf extract of B. tomentosa has shown potent anticancer activity on A549 cancerous cell line. Further studies are needed to identify the bioactive molecules and explore their mechanism of action, which might throw light on the development of new alternative drugs for lung cancer.[61,62] have reported the preparation of extracts from the various parts of B. purpurea leaves, stems, roots, and pods. These workers have isolated four new and very remarkable (dibenz[b,f]oxepins) cancer cell growth inhibitors and have designated them as Bauhiniastatins 1-4. The present study revealed that the phytochemicals in various B. variegata leaf extracts possess potent cytotoxic potential against human cancer cell lines.[63]
An EtOH extract of Bauhinia strychnifolia showed good inhibitory activity against several cancer cell lines, including HT-29, HeLa, MCF-7, and KB. Bauhinia strychnifolia stem has compounds 2 and 3 on anticancer activity. Based on the anticancer activity of extracts and pure compounds isolated from it, this plant could be helpful in the treatment of cancer.[38,64] The antiproliferative activity of specific plant lectins from seeds of Bauhinia purpurea appears to be due to the binding of lectins to the cell surface. It triggers the termination of the S and G2 phases. Induced apoptosis was found to be associated with LDH leakage, cell cycle arrest and, ROS generation. The apoptotic signal was amplified by caspase-3 activation resulting in cell death.[65-67] This study proves that P. semibfida can be used as an anticancer agent and other antidegenerative diseases with strong basic evidence based on indigenous knowledge from traditional communities. Further research is needed to explore more of its active compounds and mechanisms of anticancer action to be used as standard herbal medicines.
CONCLUSION
Panera semibifida (Roxb.) Benth. (Kangkang katup) collected from Sumatra, Indonesia, have a variety of chemical content and pharmacological activity similar to P. semibifida, which grows in different areas. The genus Phanera differs from Bauhinia based on morphological and molecular characteristics. These findings are important in authenticating herbal plants for the development of pharmacological studies. Extracts from P. semibifida are rich in flavonoids exhibiting antioxidant and antidegenerative properties such as cancer. Anticancer properties have been proven in several studies inside and outside Indonesia. P. semibfida can be used as an anticancer agent and other antidegenerative diseases with solid basic evidence based on indigenous knowledge from traditional communities.
ACKNOWLEDGEMENT
The antioxidant analysis from this study was supported by Center for Research and Development of Medicinal Plants and Traditional Medicine Tawangmangu of The Ministry and Health of The Republic of Indonesia. The authors thank all parties involved in this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
1. Yadav A, Kumari R, Yadav A. Antioxidant and its functions in human body – A review. Res Environ. Life Sci. 2016;9(11):1328-31.
2. Fitmawati F, Sofiyanti N, Roza RM, Isnaini I, Irawan YR, Winata DR, et al. Antioxidant activity of dominant plants species in Obat pahit from Lingga Malay ethnic in Riau Archipelago. Biosaintifika. 2017a;9(2):325-31. doi: 10.15294/biosaintifika.v9i2.9808.
3. Cadenas E, Packer L. Handbook of antioxidants. 2nd ed. New York: Marcel Dekker, Inc; 2002.
4. Sharifi-Rad M, Anil Kumar NV, Zucca P, Varoni EM, Dini L, Panzarini E, et al. Lifestyle, Oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front Physiol. 2020;11:694. doi: 10.3389/fphys.2020.00694, PMID 32714204.
5. Roy P, Abdulsalam FI, Pandey DK, Bhattacharjee A, Eruvaram NR, Malik T. Evaluation of antioxidant, antibacterial, and antidiabetic potential of two traditional medicinal plants of India: Swertia cordata and Swertia chirayita. Pharmacogn Res. 2015;7(Suppl 1);Suppl 1:S57-62. doi: 10.4103/0974-8490.157997, PMID 26109789.
6. Nurcahyanti AR, Arieselia Z, Kurniawan SV, Sofyan F, Wink M. Revisiting bungur (Lagerstroemia speciosa) from Indonesia as an antidiabetic agent, its mode of action, and phylogenetic position. Phcog Rev. 2018;12(23):40-5. doi: 10.4103/phrev.phrev_20_17.
7. Czerwinska ME, Bobinska A, Cichocka K, Buchholz T, Wolinski K, Melzig MF. Cornus mas and Cornus officinalis-a comparison of antioxidant and immunomodulatory activities of standardized fruit extracts in human neutrophils and caco-2 models. Plants (Basel). 2021;10(11):2347. doi: 10.3390/plants10112347, PMID 34834710.
8. Arsenov D, Župunski M, Pajevin S, Nemeš I, Simin N, Alnuqaydan AM, et al. Roots of Apium graveolens and Petroselinum crispum-Insight into Phenolic Status against Toxicity Level of Trace Elements. Plants (Basel). 2021;10(9). doi: 10.3390/plants10091785, PMID 34579318.
9. Jaikang C, Saenphet K, Sudwan P. Phytochemical screening, antioxidant and sperm viability of Nelumbo nucifera petal extracts. Laoung-on J. Plants. 2021;10(7):1375.
10. Luetragoon T, Sranujit RP, Noysang C, Thongsri Y, Potup P, Somboonjun J, et al. Evaluation of anti-inflammatory effect of Moringa oleifera Lam. and Cyanthillium cinereum (Less) H. Rob. lozenges in Volunteer Smokers. Plants (Basel). 2021;10(7):1336. doi: 10.3390/plants10071336, PMID 34208842.
11. Baratawidjaja KG. Principles of immunology. [Balai penerbit FKUI]. Jakarta; 2002.
12. Abbas AK, Lichtman AH, Pillai S. Basic immunology: Functions and disorders of the immune system. Elsevier Health Sciences; 2014.
13. Sunnitha VS, Sunil MA, Rhadhakrishnan EK, Jyothis M. Immunomodulatory Activity of Caesalpinia sappan L. Extracts on peritoneal Macrophage of albino Mice. Int J Sci Eng Res. 2015;4(12):449-52.
14. Alagawany M, Attia YA, Farag MR, Elnesr SS, Nagadi SA, Shafi ME, et al. The strategy of boosting the immune system under the covid-19 pandemic. Front Vet Sci. 2020;7:570748. doi: 10.3389/fvets.2020.570748.
15. Catanzaro M, Corsini E, Rosini M, Racchi M, Lanni C. Immunomodulators inspired by nature: A review on curcumin and echinacea. Molecules. 2018;23(11):2778. doi: 10.3390/molecules23112778, PMID 30373170.
16. Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients. 2020;12(1):236. doi: 10.3390/nu12010236, PMID 31963293.
17. Mrityunjaya M, Pavithra V, Neelam R, Janhavi P, Halami PM, Ravindra PV. Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of covid-19. Front Immunol. 2020;11:570122. doi: 10.3389/fimmu.2020.570122.
18. Lourenço SC, Moldão-Martins M, Alves VD. Antioxidants of natural plant origins: From sources to food industry applications. Molecules. 2019;24(22). doi: 10.3390/molecules24224132, PMID 31731614.
19. Costa G, Maruca A, Rocca R, Ambrosio FA, Berrino E, Carta F, et al. In silico Identification and Biological Evaluation of Antioxidant Food Components Endowed with IX and XII hCA Inhibition. Antioxidants (Basel). 2020;9(9):775. doi: 10.3390/antiox9090775, PMID 32825614.
20. Obrador E, Salvador-Palmer R, Jihad-Jebbar A, López-Blanch R, Dellinger TH, Dellinger RW, et al. Pterostilbene in cancer therapy. Antioxidants (Basel). 2021;10(3):492. doi: 10.3390/antiox10030492, PMID 33801098.
21. Londoño C, Cayssials V, De Villasante I, Crous-Bou M, Scalbert A, Weiderpass E, et al. Polyphenol intake and epithelial ovarian cancer risk in the European prospective investigation into cancer and nutrition (EPIC) study. Antioxidants (Basel). 2021;10(8):1249. doi: 10.3390/antiox10081249, PMID 34439497.
22. Khan T, Ali M, Khan A, Nisar P, Jan SA, Afridi S, et al. Anticancer plants: A review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules. 2019;10(1). doi: 10.3390/biom10010047, PMID 31892257.
23. Greenwell M, Rahman PKSM. Medicinal plants: Their use in anticancer treatment. Int J Pharm Sci Res. 2015;6(10):4103-12. doi: 10.13040/IJPSR.0975-8232.6(10).4103-12, PMID 26594645.
24. Roza RM, Isnaini IYR, Winata DR, Dewi APK. Traditional medicinal formulation: Obat pahit from Lingga Malay Ethnic in Riau Archipelago, Indonesia. Fitmawati, Sofiyanti, N. Biodiversitas. 2017b;18(3):1196-200.
25. Fitmawati F, Roza RM, Sofiyanti N, Isnaini I, Fitri FL, Paramita D, et al. Immunomodulatory effectiveness of aqueous Obat pahit ex-tract of Lingga Malay ethnic on white rats (Rattus novergicus). Biosaintifika. 2017c;9(3):430-6.
26. De Wit HCD. A revision of Malaysian Bauhinieae. Reinwardtia. 1956;3:381-539.
27. Zhang DX. A cladistic analysis of Bauhinia L. (Leguminosae). China J, (Bot). 1995;7:55-64.
28. Larsen K, Larsen SS. Bauhinia. In: Flora Malesiana HD, editor. Caesalpiniaceae (Leguminosae-Caesalpinioideae). Vol. 12(2);1996:442-535.
29. White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand D, Sninsky J, White T, editors, PCR protocols: A guide to methods and applications. San Diego: Academic Press; 1990.
30. Okonechnikov K, Golosova O, Fursov M, UGENE team. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics. 2012;28(8):1166-7. doi: 10.1093/bioinformatics/bts091, PMID 22368248.
31. Swofford DL, PAUP. *, Phylogenetic analysis using Parsimony (* and Other Methods). Versi 4.0b10. Sunderland, MA: Sinauer Associates; 2002.
32. Kress WJ, Prince LM, Williams KJ. The phylogeny and a new classification of the gingers (Zingiberaceae): Evidence from molecular data. Am J Bot. 2002;89(10):1682-96. doi: 10.3732/ajb.89.10.1682, PMID 21665595.
33. Madden T, The NCBI [handbook]. 2nd ed. Bethesda: National center for biotechnology information US; 2013.
34. Novero AU, Mabras MB, Esteban HJ. Epigenetic inheritance of spine formation in sago palm (Metroxylon sagu Roettb). Plant Omics J. 2012;5(6):559-66.
35. Sinou C, Forest F, Lewis GP, Bruneau A. The genus Bauhinia s.l. (Leguminosae): A phylogeny based on the plastid trnL–trnF region. Botany. 2009;87(10):947-60. doi: 10.1139/B09-065.
36. Hao G, Zhang DX, Zhang MY, Guo LX, Li SJ. Phylogenetics of Bauhinia subgenus Phanera (Leguminosae: Caesalpinioideae) based on ITS sequences of nuclear ribosomal DNA. Phylogenetics. Bot Bull Acad Sin. 2003;44:223-8.
37. Bruneau A, Forest F, Herendeen PS, Klitgaard BB, Lewis GP. Phylogenetic relationships in Caesalpinioideae (Leguminosae) as inferred from chloroplast trnL intron sequences. Syst Bot. 2001;26:487-514.
38. Yuenyongsawad S, Bunluepuech K, Wattanapiromsakul C, Tewtrakul S. Anti-cancer activity of compounds from Bauhinia strychnifolia stem. J Ethnopharmacol. 2013;150(2):765-9. doi: 10.1016/j.jep.2013.09.025, PMID 24120967.
39. Ferreres F, Gil-Izquierdo A, Vinholes J, Silva ST, Valentão P, Andrade PB. Bauhinia forficata Link authenticity using flavonoids profile: Relation with their biological properties. Food Chem. 2012;134(2):894-904. doi: 10.1016/j. foodchem.2012.02.201, PMID 23107705.
40. Fatima N, Baqri SSR, Alsulimani A, Fagoonee S, Slama P, Kesari KK, Roychoudhury S, Haque, S. Phytochemicals from Indian Ethnomedicines: promising prospects for the management of oxidative stress and cancer. Antioxidants. 2021;10:1606
41. Kittakoop P, Kirtikara K, Tanticharoen M, Thebtaranonth Y. Antimalarial preracemosols A and B, possible biogenetic precursors of racemosol from Bauhinia malabarica Roxb. Phytochemistry. 2000;55(4):349-52. doi: 10.1016/s0031-9422(00)00318-6, PMID 11117883.
42. Boonphong S, Puangsombat P, Baramee A, Mahidol C, Ruchirawat S, Kittakoop P. Bioactive compounds from Bauhinia purpurea possessing antimalarial, antimycobacterial, antifungal, anti-inflammatory, and cytotoxic activities. J Nat Prod. 2007;70(5):795-801. doi: 10.1021/np070010e, PMID 17480099.
43. Udomputtimekakul P, Pompimon W, Baison W, Sombutsiri P, Funnimid N, Chanadee A, et al. Profiling of Secondary Metabolites in Aerial Parts of Phanera bracteata. Am J Plant Sci. 2017;08(5):1100-34. doi: 10.4236/ajps.2017.85073.
44. Farag MA, Sakna ST, El-Fiky NM, Shabana MM, Wessjohann LA. Phytochemical, antioxidant and antidiabetic evaluation of eight Bauhinia L. species from Egypt using UHPLC-PDA-qTOF-MS and chemometrics. Phytochemistry. 2015;119:41-50. doi: 10.1016/j.phytochem.2015.09.004, PMID 26410474.
45. Idris A, Linatoc AC, Abu bakar MF, Ibrahim Takai Z, Audu Y. Effect of light quality and quantity on the accumulation of flavonoid in plant species. J Sci Technol. 2018;10(3):32-45. doi: 10.30880/jst.2018.10.03.006.
46. Panche AN, Diwan AD, Chandra SR. Flavonoids: An overview. J Nutr Sci. 2016;5:e47. doi: 10.1017/jns.2016.41, PMID 28620474.
47. Tanjung M, Tjahjandarie TS. Flavonoids from the stem bark of Bauhinia semibifida L. Scholar Res Lib. 2014; 6 (6):434-8.
48. Kumar S, Mishra A, Pandey AK. Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Complement Altern Med. 2013;13:article 120. doi: 10.1186/1472-6882-13-120, PMID 23721571.
49. Adisai SD, Tinpun K, Wattanapiromsakul C, Keawpradub N. Bioactivities and phytochemical investigation of Cnestis palaeola (Lour) Merr. Afr J Trad Complement Altern Med. 2015;12(3):27-37.
50. Fadhli H, Furi M, Jauwahir A. Isolation and antioxidant activity testing alkaloids from stem bark methanol extract Bunga Kupu-kupu (Bauhinia semibifida Roxb.). J P farmasi Ind. 2019;7(2):43-50.
51. Zuhra CF, Tarigan J, Sihotang H. Antioxidant activity of flavonoid from katuk (Sauropus androgynus (L.) Merr.) leaves. Jurnal Biologi Sumatera. 2008;3(1):7-10.
52. Sashidara KV, Singh PS, Misra S, Gupta J, Bhattacharya SM. Galactolipids from Bauhinia racemosa as a nes class of antifilarial agents against human lymohatuc filarial prasites. Brugia malayi J Ejmech. 2012. Vol. 50. PMID 230235.
53. Souza JD, Silva MBR, Argolo ACC, Napoleão TH, Sá RA, Correia MTS, et al. A new Bauhinia monandra galactose-specific lectin purified in milligram quantities from secondary roots with antifungal and termiticidal activities. Int Biodeterior Biodegrad. 2011;65(5):696-702. doi: 10.1016/j. ibiod.2011.02.009.
54. Tiwari V. Pharmacognostical, phytochemical, antimicrobial evaluation of Bauhunia tementosa. Jpr. 2011;4(4):1173-5.
55. Rajkapoor B, Jayakar B, Murugesh N, Sakthisekaran D. Chemoprevention and cytotoxic effect of Bauhinia variegata against N-nitrosodiethylamine induced liver tumors and human cancer cell lines. J Ethnopharmacol. 2006;104(3):407-9. doi: 10.1016/j.jep.2005.08.074, PMID 16257158.
56. Gupta M, Mazumder UK, Kumar R, Sambath GP, Rajeshwae Y, Kakoti BB, et al. Antiinflamantory, analgetic, and antipyretic effects of methanol extract from Bauhinia racemosa stem bark in animal models, J Ethpharm. 2005;98:267-73.
57. Saroj P, Verma M, Jha K, Pal M. An overeview on immunomodulation. Adv Sci Res. 2012;3(1):7-12.
58. Erdityo CW. Isolation and identification fenolic compound from Bauhinia sembifida stem and anticancer activity to Murine cell Leukimia P-38 [thesis]. Universitas Airlangga; 2014.
59. Chew YL, Lim YY, Stanslas J, Ee GCL, Goh JK. Bioactivity-guided isolation of anticancer agents from Bauhinia kockiana Korth. Afr J Tradit Complement Altern Med. 2014;11(3):291-9. doi: 10.4314/ajtcam.v11i3.40, PMID 25371595.
60. Pandey S. In vivo antitumor potential of extracts from different parts of Bauhinia variegata linn. Against b16f10 melanoma tumour model in C57BL/6 mice. Appl Cancer Res. 2017;37(1):33. doi: 10.1186/s41241-017-0039-3.
61. Balabhaskar R, Vijayalakshmi K. Evaluation of Anticancer activity of ethanol extract of Bauhinia tomentosa Linn. on A549, Human Lung Carcinoma Cell lines. Res J Pharm Technol. 2019;12(6):2748-52. doi: 10.5958/0974-360X.2019.00460.8.
62. Pettit GR, Numata A, Iwamoto C, Usami Y, Yamada T, Ohishi H, et al. Antineoplastic agents. 551. Isolation and structures of bauhiniastatins 1-4 from Bauhinia purpurea. J Nat Prod. 2006;69(3):323-7. doi: 10.1021/np058075+, PMID 16562827.
63. Mishra A, Sharma AK, Kumar S, Saxena AK, Pandey AK. Bauhinia variegata leaf extracts exhibit considerable antibacterial, antioxidant, and anticancer activities. BioMed Res Int. 2013;2013:915436. doi: 10.1155/2013/915436.
64. Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, et al. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48(3):589-601. PMID 3335022.
65. Agrawal SB, Gupta N, Bhagyawant SS, Gaikwad SM. Anticancer activity of lectins from Bauhinia purpurea and Wisteria floribunda on breast cancer MCF-7 cell lines. Protein Pept Lett. 2020;27(9):870-7. doi: 10.2174/0929866527666200 408143614, PMID 32268858.
66. McComb S, Chan PK, Guinot A, Hartmannsdottir H, Jenni S, Dobay MP, et al. Efficient apoptosis requires feedback amplification of upstream apoptotic signals by effector caspase-3 or -7. Sci Adv. 2019;5(7):eaau9433. doi: 10.1126/sciadv.aau9433, PMID 31392262.
67. Dutordoir MR, Bates DAA. Activated of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;2977-92:1863;12.
Cite this article: Fitmawati, Juliantari E, Roza RM, Titrawani, Yahya VJ. Reactualiting Phanera semibifida (Roxb.) Benth. from Sumatra, Indonesia as an Anticancer Agent and Phylogenetic Position. Pharmacog Rev. 2022;16(31):34-9.