|
Pharmacogn Rev. 2022;16(31):12-21 A multifaceted peer reviewed journal in the field of Pharmacognosy and Natural Products www.phcogrev.com | www.phcog.net |
Review Article |
The use of Curcuma longa and its Derivatives in the Treatment of Osteorthritis: A Scoping Review
José Fernando de Araújo Neto*, Juliana Azevedo da Paixão, Ademir Evangelista Do Vale, Jorge Mauricio David, Juceni Pereira De Lima David
José Fernando de Araújo Neto*, Juliana Azevedo da Paixão, Ademir Evangelista Do Vale, Jorge Mauricio David, Juceni Pereira De Lima David
Universidade Federal da Bahia, Institute of Chemistry, Salvador, BRAZIL.
Correspondence
Prof. José Fernando de Araújo Neto,
Universidade Federal da Bahia, Institute of Chemistry, Salvador, BRAZIL.
E-mail: [email protected]
History
• Submission Date: 01-09-2021;
• Review completed: 13-10-2021;
• Accepted Date: 24-11-2021.
DOI : 10.5530/phrev.2022.16.3
Article Available online
http://www.phcogrev.com/v16/i31
Copyright
© 2022 Phcog.Net. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International license.

ABSTRACT
Osteoarthritis is considered a degenerative disease, with knee and hip osteoarthritis being the most common form. Drug treatment is aimed at relieving symptoms with analgesics and non-selective or selective NSAIDs. Curcuma longa is a species used in Ayurvedic medicine without treating inflammatory conditions. The purpose of this scoping review is to identify and examine the evidence related to the effects of using C. longa in the treatment of osteoarthritis and the possible benefits in relation to treatment with NSAIDs. This research was conducted using an approach proposed by Arksey and O’Malley, the JBI Scope Review Methodology Handbook, and the PRISMA-ScR Guidelines and Checklist. Four studies meet the inclusion criteria, three were obtained as trials randomized clinical trials and the fourth study is a pilot clinical trial. The evidence raised shows that treatment with C. longa can reduce the symptoms of osteoarthritis with less potential to cause adverse events. However, the methodological quality of the included studies and the sample size do not allow definitive selections to be taken. These limitations indicate the need to carry out randomized clinical trials of high methodological quality, following CONSORT guidelines to confirm the efficacy of C. longa in the treatment of osteoarthritis and its benefits over conventional treatments.
Key words: Curcuma longa, Curcuma domestica, Osteoarthritis, Curcuminoids, Nonsteroidal Anti-inflammatory Drugs.
Cite this article: Neto JFA, Paixão JA, Vale AED, David JM, David JPDL. The use of Curcuma longa and its Derivatives in the Treatment of Osteorthritis: A Scoping Review. Pharmacog Rev. 2022;16(31):12-21.
INTRODUCTION
The two most common types of arthritis are osteoarthritis (OA) and rheumatoid arthritis (RA). Osteoarthritis is generally considered to be a degenerative disease which involves the progressive destruction of articular cartilage in synovial joints and remodeling of the adjacent bone,[1] although in some aspects it can be seen as an inflammatory disease.[2-5]
Osteoarthritis is a progressive and slow musculoskeletal disorder, generally insidious, that typically affects the joints of the hands, spine, hip and knee. OA primarily characterized by pain and mechanical deterioration of the joint, progressively leads to limited movement (difficulty walking and climbing stairs) and functional disability. The process involves cartilage degeneration, proliferation and remodeling of the subchondral bone structure. Osteoarthritis is a multifactorial disease that causes damage to the articular cartilage and inflammatory changes in the joint.[1,6] It is a slow and gradual process, highly prevalent in the adult population, which causes pain, loss of function and quality of life, especially in the elderly and obese.[7] Clinically, OA is characterized by pain, morning stiffness, bone crackling, muscle atrophy and as regards the radiological aspects, narrowing of the intra-articular space, osteophyte formations, subchondral bone sclerosis and cystic formations.[8,9]
Osteoarthritis of the knee and hip is the most common form of arthritis. It has become an increasing health concern.[10,11] Pain and illness can vary from very mild to very severe.[11] OA patients have pain that usually gets worse with weight, walking and standing, and improves with rest. Other symptoms include morning stiffness and articular gelling after periods of inactivity.
There are two types of osteoarthritis; considering the etiology, the primary, with unknown initial cause, or the secondary, when it is triggered by known and determined factors. Secondary OA can occur after arthritis, trauma and osteonecrosis, for example.[8] Some patients have an erosive OA, which can become more severe with inflammatory signs and major deformities. On physical examination, pain on palpation indicates joint enlargement. An increase in joint temperature and stroke are consequences of an inflammatory crises.[12] In primary or secondary OA, cartilage is the tissue with the greatest changes.[9] Among the morphological changes, the articular cartilage loses its homogeneous nature and is ruptured and fragmented, with fibrillation, fissures and ulcerations.[8] Sometimes, as the pathology advances, the cartilage thickness is lost and areas of subchondral bone are exposed.[7,9]
The diagnosis of knee osteoarthritis is clinical-radiological. In general, symptoms and signs are pain, mobility limitations, crackling, joint effusion and deformities, however, these changes are nonspecific and may be present in other conditions, such as inflammatory joint diseases. Thus, in order to obtain a diagnosis of knee osteoarthritis, the existence of reactive degenerative changes, such as the occurrence of osteophytes and / or decreased of cartilage thickness, must be verified.[13]
Osteoarthritis affects about 12% of the population worldwide,[1] and its prevalence is increasing due to an aging population and increasing rates of obesity.[14] Hip and knee osteoarthritis was recently classified as the 11th cause of global disability,[15] with an enormous economic burden.
The World Health Organization estimates that 10% of the population over 60 years old has serious medical problems resulting from OA, it is the fourth disease that most reduces the quality of life for each year lived. Furthermore, individuals with arthrosis report a significant loss in the development of their tasks, where continuous pain and limitations prohibit them from performing simple activities.[16]
The various clinical patterns of osteoarthritis, observed in approximately 10% of people over 60 years old, interferes with the quality of life of millions of Americans. In addition, the estimated costs of spending on OA are between 0.25% and 0.50% of a country’s gross domestic product, and the average annual direct and indirect costs are approximately US$7000 per person.[17]
The aims of the OA treatment approach are, in general, the education of the patient about the disease, its control; pain control; improving function and decreasing disability; and the change in the disease process and its consequences. Non-pharmacological attitudes such as patient education, weight loss and physiotherapy are the first line of treatment, which have low cost and little potential for complications and play a fundamental role in the treatment of OA. Drug treatment, on the other hand, can be of systemic, topical or intra-articular use aimed at the treatment of symptoms, as in the case of non-selective or selective non-steroidal or selective anti-inflammatory drugs for COX-2. This treatment can also aim to modify symptoms and/or evolution of the disease. The first choice medication for pain control is paracetamol, at a dose of up to 4 g/day.[18]
Current osteoarthritis treatment includes exercise, heat/cold therapy, joint protection, weight loss, physical therapy/occupational therapy in addition to drug treatment.[11,19] The main objective of drug treatment is the relief of symptoms such as pain and inflammation. Current clinical guidelines recommend treating OA with over-the-counter analgesics such as paracetamol and NSAIDs, however there are considerable side effects associated with the use of these medications.[20-24] Other treatment options include specific cyclooxygenase-2 inhibitors, intra-articular injections of corticosteroids, tramadol and other opioid analgesics to relieve severe pain.[20] Intra-articular injection of hyaluronic acid is another therapeutic approach for knee osteoarthritis that does not respond to conventional measures in patients who wish to delay or avoid knee replacement surgery.[3,25,26] Although these therapies may relieve symptoms in the short term, their final impact on the pathophysiological progression of OA is limited.[27] Understanding the pathophysiology of osteoarthritis provides a more rational opportunity to define targets for pharmacological treatment that are involved in the degenerative process.
Osteoarthritis sufferers use many nutraceuticals to relieve pain and discomfort. Nutraceuticals defined as functional foods, natural products or parts of foods provide medicinal, therapeutic or health benefits, including disease prevention or treatment. These products are commonly used because they are well tolerated and considered safe. Currently, 69% of patients with OA use some type of dietary supplement for their condition.[28] Use regularly occurs in conjunction or as an alternative to first-line interventions, such as exercise. This behave results in spending on alternative therapies for the treatment of OA almost equal to spending on traditional pharmacological therapy.[29]
The genus Curcuma is represented by a group of herbaceous plants native to tropical and subtropical regions.[30] Curcuma longa (sin. C. domestica) is a species from Zingiberaceae family cultivated in India and other parts of Asia. The rhizome is the most used part of the plant, being used by Ayurvedic medicine to treat inflammatory conditions.[31]
The main active components of the C. longa rhizome are curcuminoids (curcumin, demetoxicurcumin and bisdemetoxicurcumin) and the volatile oil containing tumerone, atlantone and zingiberone, in addition to sugars, proteins and resins.[32-34] Curcuminoids are non-volatile polyphenolic compounds derived from curcumin that have a variety of biological activities.[35]
In traditional medicine, C. longa is used as a carminative, stomaquic, digestive, anthelmintic, tonic and laxative.[36] It is also used to treat fever, gastritis, dysentery, infections, chest congestion, cough, hypercholesterolemia, hypertension, rheumatoid arthritis, jaundice, liver and gallbladder problems, urinary tract infections, skin diseases, diabetic wounds and menstrual discomfort.[37-39]
The purpose of this scope review is to identify and examine the evidence related to the effects of using Curcuma longa in the treatment of osteoarthritis and the possible benefits in relation to treatment with NSAIDs, in order to support evidence-based practice.
MATERIALS AND METHODS
This research was carried out using the approach of a Scope review, following the structure proposed by Arksey and O’Malley.[40] A scope review maps the sources and types of evidence existing in a field of interest, summarize, and disseminate research results. Our method was guided by several resources, including the Scope review methodology manual published by the Joanna Briggs Institute,[41] the instrument for the critical evaluation of controlled and randomized clinical trials published by the Joanna Briggs Institute and the PRISMA-ScR checklist, which is an extension of PRISMA, this checklist provides clear guidelines for conducting and writing Scope reviews.[42] Scope reviews involve the review and collection of publicly available data and information and, therefore, approval of research by an ethics committee for research with humans was not required.
Participants (Population)
Studies with adults over 40 years of any gender with a confirmed diagnosis of knee osteoarthritis through clinical evidence using extract of Curcuma longa or its derivatives compared to the treatment with NSAIDs.
Studies in which the treatment consisted of the association of Curcuma longa or its derivatives with other extracts or substances and studies whose comparator was placebo or another treatment other than NSAIDs, as well as Studies without full texts available in English or Portuguese, were excluded.
Concept
This review characterized the relevant concepts in relation to the interventions of interest for this research. The use of Curcuma longa extract or its curcuminoid / curcumin derivatives in the treatment of knee osteoarthritis, including all forms of administration, doses and dosage compared to treatment with NSAIDs. Studies that used Curcuma longa extract or its derivatives associated with extracts from other plant species or substances were excluded.
Context
The eligible studies were those that focused on comparing the treatment of knee osteoarthritis with Curcuma longa or its curcuminoid / curcumin derivatives with treatment with NSAIDs in English and Portuguese, from the year 2000 to the present. This period allows a greater variety of evidence to be collected that reflects the contemporary nature of the topic.
Sources and types of Evidence
This study included randomized clinical studies and nonrandomized clinical studies that investigated the use of Curcuma longa or its curcuminoid / curcumin derivatives in the treatment of knee osteoarthritis compared to treatment with NSAIDs. Editorials, letters, comments, abstracts, case reports, observational studies, qualitative studies, narrative reviews and systematic reviews were excluded.
Information Sources and Search Strategy
The search strategy conducted in February / 2020 was initially throught PubMed and then adapted for the Cochrane Library and Scielo databases (Supplementary Material for the Search Strategy). The original research used the following keywords: Curcuma, Curcuma longa, Curcuma zedoaria, curcuminoids, curcumin, Turmeric, osteoarthritis, osteoarthritides, osteoarthrosis, and osteoarthroses. The initial analysis involved reading the titles and abstracts of the studies found. It was necessary read the selected studies in full to determine whether they met the inclusion / exclusion criteria.
The search in the gray literature also conducted in February 2020 used the search strategy “Curcuma longa” and “osteoarthritis” and “Randomized Control Trial” or “Non-Randomized Control Trial” in Google Scholar. to search for other works not previously identified we used the reference lists of the included studies. The published literature included in the study was summarized in a data extraction table (Supplementary Material of the Included Studies).
Methodological Quality Assessment
Although PRISMA-ScR does not require an assessment of the methodological quality of the evidence, this review carried out an assessment of the methodological quality to allow for a structured and critical examination of the characteristics of the evidence.
Data Extraction
A standardized data extraction tool (Supplementary Material of the Included Studies) was used to collect the following information:
Study data: study authors; year of publication; country of study or, if not reported, country of first author; funding source; study design; objective; and sample size.
Population demographic data: proportion of men / women / other participants, age distribution and mean, age-related inclusion criteria, race / ethnicity distribution, population data on physical health comorbidities (eg, chronic pain, hypertension, diabetes and cancer).
Type of intervention: Curcuma longa extract / curcuminoids / curcumin, concentration, pharmaceutical form, dosage and dose.
Comparator: comparison with non-steroidal anti-inflammatory drugs.
Outcomes: For each reported outcome of interest, we defined the meaning of the result, duration of follow-up, direction of effect and significance. Since this is a scope review, all results of interest had the same priority.
Summary and Presentation of Data
The data synthesized based on the objective of the study, related to the treatment with Curcuma longa or its derivatives and to any reported effect in relation to osteoarthritis. The mapping of the included evidence conducted in Microsoft Excel (Office 365), organizing the results in tables, can allow the identification of comparisons between the types of study design, in addition to informing the identification of contradictory results, if present. In addition to presenting the data in tables, the results were described through a narrative summary of the extracted information. Any trends or patterns identified were reported in the results. The final report of the scope review followed the items described in the Prisma-ScR.
RESULTS
The adapted PRISMA flowchart (Figure 1) shows the results of the search and the analysis process of the studies found (n = 337) in relation to the inclusion / exclusion criteria. Nine articles excluded because they were duplicates. Most of the articles examined focused on the use of Curcuma longa extract or its derivatives and osteoarthritis, but referred to in vitro or animal studies or were review studies on OA or Curcuma longa, these studies were excluded from from reading the title and abstract (n = 316).
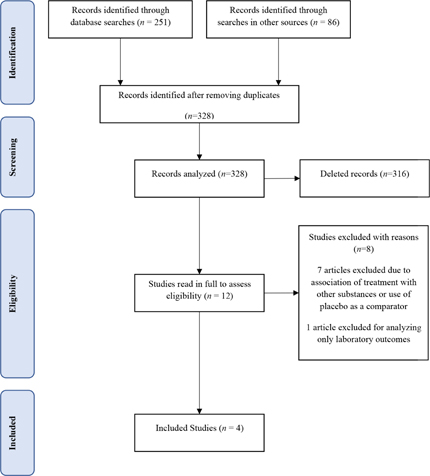
Figure 1: PRISMA Flowchart.
The 12 selected articles were read in full and four studies were included in this scope review. Seven articles excluded after reading the text, performed the treatment with Curcuma longa and its derivatives in association with other substances or compared with placebo; one article excluded because only laboratory outcomes were analyzed.
This resulted in four studies focused on comparing treatment with Curcuma longa extract and its derivatives with treatment with NSAIDs for osteoarthritis. There were three randomized studies, being a double-blind multicentre, a double blind and a single blind open randomized study. The fourth study was a pilot clinical study.
Included Studies
All included studies that met the inclusion criteria analyzed the effectiveness of Curcuma longa extract in treating osteoarthritis compared to NSAIDs.[43-46] Three have been described as randomized controlled trials,[43-45] however, only two have registered the trial, one as a double-blind trial,[44] and the other as a single-blind trial.[45] The fourth study was registered as a randomized double-blind clinical trial,[46] but the results presented refer to a pilot clinical study with 42 participants. Two studies were carried out in India,[45,46] and the other two in Thailand.[43,44]
The four studies used different treatment regimens with extracts of Curcuma longa to treat OA. In one study, gelatin capsules containing 500 mg of turmeric extract (each capsule contained at least 88% curcuminoids and 68% curcumin) were used, the dosage was one capsule three times a day for 28 days.[45] The dosage in another study was two capsules after meals, three times a day for 4 weeks, each capsule containing 250mg of curcuminoids.[44] The 2000 mg daily dose was used in another study, each capsule contained 250 mg of curcuminoids, the dosage being determined at 500 mg four times a day for 6 weeks.[43] The study with the lowest daily dose used 160 mg of curcumin per day divided into two daily doses of 80 mg after meals with water for a period of 90 days. The lower dose is justified due to the encapsulation of curcumin in a lipid complex that increases solubility.[46]
In relation to the comparator, in three studies ibuprofen was used in the doses of 400,[46] 800,[43] and 1200 mg per day.[44] In the fourth study, the use of diclofenac occurs at a daily dose of 100 mg, divided into two doses per day. Find the main objectives and interventions of each study described in the Supplementary Material of the Included Studies.
Methodological Quality of Included Studies
The methodological assessment of the quality of the four included studies was carried out using the Joanna Briggs Institute’s Critical Assessment Tool for Randomized Controlled Studies,[47] composed of 13 items. Find the information described in a critical analysis table (Table 1). The studies carried out by Shep et al. (2019), Gupte et al. (2019) and Kuptniratsaikul et al. (2009) satisfied nine of these 13 items,[43,45,46] The study by Kuptniratsaikul et al. (2014) satisfied eleven items.[44]
Table 1: Critical analysis of included studies.
| Included studies | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shep et al. (2019) | Y | Y | Y | N | NC | N | Y | Y | N | Y | Y | Y | Y | 09/13 |
| Gupte et al. (2019) | Y | Y | N | Y | NC | NC | Y | Y | N | Y | Y | Y | Y | 09/13 |
| Kuptniratsaikul et al. (2009) | Y | NC | Y | NC | NC | Y | Y | Y | N | Y | Y | Y | Y | 09/13 |
| Kuptniratsaikul et al. (2014) | Y | Y | Y | Y | Y | Y | Y | N | N | Y | Y | Y | Y | 11/13 |
Y = Yes, N = No, NC = Not clear
Critical Evaluation Instrument for Randomized Controlled Studies JBI
Q1 Was the allocation of participants to treatment groups truly random?
Q2 Was allocation to groups blinded?
Q3 Were treatment groups comparable at baseline?
Q4 Was the assignment of treatment hidden from participants?
Q5 Was it hidden from those responsible for administering the treatment which group the participants were allocated to?
Q6 Was the group to which the participants were allocated hidden from the outcome assessors?
Q7 Were the different study groups treated identically, with the exception of the referred intervention?
Q8 Was follow-up completed? If it was not, were the differences between groups at follow-up adequately described and analyzed?
Q9 Were participants analyzed in the groups to which they were randomized?
Q10 Were the results evaluated in the same way for all groups?
Q11 Were the results measured reliably?
Q12 Was an appropriate statistical analysis used?
Q13 Is the study design appropriate to the topic under review, and was there any evidence of deviation from the standard design of an RCS during the development or review phases?
All included studies reported appropriate randomization techniques. Three studies described procedures for blinding the allocation of patients,[44-46] only the study by Kuptniratsaikul et al. (2009) did not report whether blinding was performed in the allocation of patients.[43] The study by Shep et al. (2019), an open randomized parallel group study, ensured that participants and raters were aware of the treatment regimen. In the study by Gupte et al. (2019), the participants were blind, but it is not clear whether the evaluators and those responsible for applying the treatment were aware of the treatment regimen or not. Despite the study by Kuptniratsaikul et al. (2009) report that only one researcher not directly associated with the study knew what the treatment regimen of the patients was, the study only describes that the evaluators of the outcomes were not aware of the treatment allocated to each patient. It did not described in the study which procedure was used to blind patients and those responsible for delivering treatment to patients. The study by Kuptniratsaikul et al. (2014) described the procedures performed to blind patients and researchers about the treatment regimen.
The groups of treatment and control had similar characteristics in three studies.[44,45] In one of the studies, there was a significantly more women in the control group, while the number of patients with normal weight and comorbidities was higher in the treatment group.[46] There was no difference regarding the care received by the control and treatment groups, with the exception of the intervention.
The studies carried out by Kuptniratsaikul et al. (2009) and Kuptniratsaikul et al. (2014) decribed calculations performed to determine the sample size needed to perform a non-inferiority study with per-protocol analysis. In the first study, amoung the 52 patients in the treatment group 7 (13.5%) did not complete. While in the control group 9 patients (16.4%) amoung the 55 did not complete, 6 patients in each group abandoned the study for not returning to follow-up visits and three patients dropped out due to adverse events (1 in the treatment group and 2 in the control group).[43] In the study carried out in 2014, in the sample size calculated for a non-inferiority design with analysis by protocol, amoung 367 patients participated in the study, 185 in the treatment group and 182 in the control group, 22 patients did not complete treatment in the control group and 14 in the treatment group. Twelve patients in the control group and 9 patients in the treatment group were removed from the study, but the reasons were not reported. In the control group, it was not possible to make contact with three patients, while 7 left the study due to the occurrence of adverse events. In relation to the treatment group, it was not possible to make contact with three patients, one patient left the study due to the occurrence of adverse events and one of them due to inconvenience.[44]
Supplementary Material of the Included Studies
| Reference | Year of Publication | Country | Financing | Desenho do Estudo | Aim | Sample size | Inclusion criteria |
|---|---|---|---|---|---|---|---|
| [48] | 2009 | Thailand | Thai Traditional Medicine and Alternative Medicine Development Department, Ministry of Public Health and Thailand Research Fund | Randomized controlled study in a university hospital in Bangkok, Thailand. | To determine the efficacy and safety of C. domestica extracts in pain reduction and functional improvement in patients with knee osteoarthritis. | 190 patients selected and 107 met the selection criteria and were included in the study, of which 52 were randomized to the C. domestica group and 55 to the ibuprofen group, respectively. 45 patients completed the study in the C. domestica group and 46 in the ibuprofen group. | Adults with primary knee OA, according to American Rheumatism Association criteria. The individual should have knee pain and radiographic bone spurs and at least one of the following characteristics: (1) age >50 years, (2) morning stiffness lasting <30 min, (3) and crackling in motion. Patients who had a pain score on the numerical rating scale ≥5 of 10 recruited. |
| [49] | 2014 | Thailand | National Research Council of Thailand (NRCT), the Clinical Research and Collaboration Network (CRCN) and the Pharmaceutical Organization of the Government of Thailand | Randomized, multicentre, double-blind clinical trial conducted in 8 tertiary hospitals across Thailand between July 2010 and March 2012 | To determine the efficacy and safety of C. domestica extract in pain reduction and functional improvement compared to ibuprofen. | 524 patients with knee OA examined and 367 recruited, of which 182 individuals were randomized to the ibuprofen group and 185 to the C. domestica group. 160 patients in the ibuprofen group and 171 in the C. domestica group completed the study. | Patients with primary knee osteoarthritis, according to the American Rheumatism Association criteria, who had a numerical knee pain scale ≥5 out of 10 and age ≥50 years. |
| [50] | 2019 | India | Not reported | Prospective, randomized, parallel group, open, controlled study.
Study conducted at City Care Accident Hospital, Parli Vaijnath, and Maharashtra, India. |
To compare the efficacy and safety of curcumin with that of diclofenac in patients with knee osteoarthritis. | 160 patients screened and 149 patients included in the study. | Patients (aged 38 to 65 years) with symptomatic knee osteoarthritis for at least 3 months without joint deformities and requiring treatment with anti-inflammatory drugs. |
| [51] | 2019 | India | Pharmanza Herbals Pvt. Ltd | Pilot clinical study | To evaluate the efficacy and safety of SLCP (curcumin optimized for 160 mg/day) in patients with knee osteoarthritis. | 2 patients, 25 in the control group and 17 in the study group. There were two dropouts in the control group and five in the study group. | Patients of both sexes, aged between 40 and 65 years, suffering from knee joint osteoarthritis based on the American College of Rheumatology (ACR) criteria, willing to comply with the study protocol. |
Supplementary Material of the Included Studies
| Reference | Proportion of Men/Women | Mean age ± SD (years) | Treatment | Control | Outcomes |
|---|---|---|---|---|---|
| [48] | Female gender (%):
C. domestica Group 41 (78.8%) Ibuprofen Group 45 (81.8%) |
C. domestica Group: 61.4 ± 8.7
Ibuprofen Group: 60.0 ± 8.4 |
Extract of C. domestica (500 mg four times a day) for 6 weeks. Patients instructed not to use other medications or herbs. Each capsule of C. domestica extract contained 250 mg of curcuminoids. | Ibuprofen (400mg twice daily) for 6 weeks (800mg daily) | Pain on level walking and pain when climbing stairs assessed by a numerical rating scale and knee function assessment by the time spent on a 100 m walk up and down a flight of stairs (10 steps). |
| [49] | Female gender (%):
Curcuma Group 157 (91.8%) Ibuprofen Group 139 (86.9%) |
Curcum Group: 60.3 ± 6.8
Ibuprofen Group: 60.9 ± 6.9 |
1500 mg per day of curcumin extract. The curcuminoid extract prepared from the rhizome had a total curcuminoid content between 75% and 85%, each capsule contained 250 mg of curcuminoids. Patients asked to take the drug as two capsules after meals, three times a day for 4 weeks and not to use other drugs during the study. If they had severe pain, they could take tramadol as a rescue medication. | 1200 mg per day of ibuprofen | Outcomes assessed using the modified Thai version of the WOMAC index and a 6-min walk. |
| [50] | Gender (M/F):
Curcumin Group 45 / 25 Diclofenac Group 48 / 21 |
Curcumin Group: 53.09 ± 4.17
Diclofenac Group: 52.14 ± 3.76 |
500 mg Curcumin (BCM-95®) Hard Gelatin Capsule Size Zero (Curcugreen®, Arjuna Natural Ltd., Kerala, India). Each capsule contained curcuminoids and turmeric complex essential oil (curcumin, desmethoxycurcumin, and bisdemethoxycurcumin and turmeric rhizome volatile oils) in a concentration not less than 95%, at least 88% curcuminoids and 68% curcumin. The dosage of curcumin 500 mg was three times a day for 28 days. | Uncoated diclofenac 50 mg tablet (Lupin Pharmaceuticals, Mumbai, India). The dosage of diclofenac 50 mg was twice daily for 28 days. | Primary outcomes assessed using the VAS scale. Secondary outcomes: improvement in pain intensity on the KOOS subscale, anti-flatulent effect, weight-increasing effect, patient’s global assessment for overall symptom relief, physician’s global assessment of treatment, and anti-ulcer effect. |
| [51] | Sexo (M/F):
Study Group 6(35%) / 11(64.7%) Control Group 2(8%) / 23(92%) |
Control Group = 54 ± 8
Study Group = 57 ± 7.5 |
SLCP in capsule form twice daily (80 mg/capsule). After meals with water for a period of 90 days. Each capsule is a patented 400 mg lipophilic matrix providing 80 mg of curcumin, manufactured using SLCP™ technology. | Ibuprofen capsule (400 mg) once in the morning, followed by placebo (dextrin) in the evening after meals for 90 days. | The primary outcome was pain reduction assessed using the WOMAC index, while the secondary was the change in inflammatory and degenerative markers. Patients’ perception of pain documented in the Visual Analog Scale (VAS). In addition to the assessment of VAS and WOMAC, other signs and symptoms such as crepitation, swelling, increased temperature (indicating acute inflammation) and degree of flexion (using a goniometer) observed. |
The study by Gupte et al. (2019) did not perform calculations to determine the sample size; the study analysis was performed by protocol. Due to this study is a pilot study, the number of participants was reduced when compared to the other studies, there were 50 patients in total, 27 in the control group and 23 in the treatment group, two losses occurred in the control group (7.4%) and 6 in the treatment group (26.1%). Two patients left the study in the treatment group due to nausea and heartburn and one patient due to skin rash and itching all over the body, the other patients abandoned the study because they did not return to follow-up visits. Despite the difference in the number of patients who did not complete the study, the analysis of efficacy revealed that there were no differences between groups.[46]
The study by Shep et al. (2019) performed calculations to determine the required sample size, although the study reports in the methodology that analyzes performed would be of the intention-to-treat type, the results presented considered only the patients who completed the study in each group. They allocated 74 patients to the treatment group and 75 patients to the control group. Four patients left the study in the treatment group, 3 were lost during the follow-up and one stopped taking the medication for more than a day, in the control group six patients left the study, 4 were lost during the follow-up and 2 had gastrointestinal adverse events.[45]
Demographic Data of Participants
The four included studies described the characteristics of the participants and risk factors related to osteoarthritis such as age, gender, body mass index, in addition to the duration of symptoms and the intensity of pain from knee osteoarthritis. In all studies, age groups were evenly distributed in the control and treatment groups.
Treatment Results
The main symptoms of osteoarthritis are pain and inflammation. The symptoms can be classified as pain, stiffness, swelling and difficulty in movement. One of the ways to assess the functional improvement of a patient diagnosed with OA is with standardized questionnaires where the subject reports his difficulties. Most studies evaluated as outcomes the severity of OA symptoms according to pain intensity, stiffness and function, being mainly sized by the VAS and WOMAC scales. Three studies used standardized questionnaires to assess treatment outcomes for osteoarthritis.[44-46] In only one study, a different method was used to assess outcomes related to the presence of pain in level gait and stairs and the time spent on a 100m walk and to go up and down a flight of stairs.[43]
The study performed by Kuptniratsaikul et al. (2014) showed that the main outcomes were assessed using a modified Thai version of the WOMAC index and by the distance walked on foot for 6 min at the beginning and after the second and fourth week of treatment. The WOMAC index divided into three subscales ranging from 0 to 10, which correspond to pain, stiffness and function. The higher the index, the more pain, stiffness or worse the function of the knee. There was a reduction in the WOMAC index in both groups after four weeks, with no significant differences between the treatment and control groups. Treatment with C. domestica proved to be non-inferior to treatment with ibuprofen in reducing the total WOMAC, pain and function indices. In the WOMAC index for stiffness, treatment with ibuprofen was superior to treatment with C. domestica. Considering the 6-min walk, there was no difference between the groups.[44]
In the study by Gupte et al. (2019) the outcomes were assessed using the WOMAC index at the beginning and after 30, 60 and 90 days of treatment and through the Visual Analogue Scale (VAS), which assesses the patients’ perception of pain, at the beginning and after 7, 15, 45, 75 and 90 days of treatment. There was a significant improvement in the VAS index in both groups from day 45. The WOMAC index gradually decreased, being statistically significant compared to baseline for the two groups from day 60, with no significant difference between the two groups.[46]
In the study by Shep et al. (2019), patients were assessed at the beginning and after the second and fourth week of treatment. Using the Visual Analogue Scale (VAS) and through the KOOS Scale, a scale used to assess the intensity of pain in knee injuries that is divided into five items: pain, symptoms, function in daily life, function in sports and recreation and quality of life. The groups of treatment and control showed a significant reduction in the VAS scale in relation to the beginning of treatment, however, there was no significant difference in the reduction of pain intensity between the groups. There was a statistically significant improvement in all five KOOS subscales, with no difference between the group that received curcumin and the group that received diclofenac.[45]
Only in the study by Kuptniratsaikul et al. (2009) the outcomes were assessed through pain when walking on a level surface and pain when walking on stairs using a numerical rating scale and through the evaluation of knee function according to the time spent on a 100m walk and to climb and go down a flight of stairs (10 steps). The results in each group were significantly better at the sixth week when compared to baseline values, showing a decreasing trend. Although there was no statistical difference between the groups, the group C. domestica spent less time to walk 100m and to go up and down a flight of stairs.[43]
Adverse events
Few significant adverse events reported in the studies that used ibuprofen as a comparator[43,44] the occurrence of adverse events was lower in the treatment group, the most common adverse events were related to problems in the gastrointestinal tract.
In the study by Kuptniratsaikul et al. (2009), the occurrence of adverse events was lower in the C. domestica group (33.3%) when compared to the ibuprofen group (44.2%); however, there was no statistical difference between the groups. The most common adverse events in the C. domestica and ibuprofen groups were dyspepsia, dizziness, nausea, vomiting and diarrhea.[43] Results similar to those found by Kuptniratsaikul et al. (2014). The occurrence of adverse events was not statistically different between the two groups: 35.7% in the ibuprofen group and 29.7% in the C. domestica group. The most common adverse events were dyspepsia, abdominal pain / distension, nausea, diarrhea and cutaneous edema. The occurrence of abdominal pain / distension was significantly lower in the C. domestica group than in the ibuprofen group. Despite the occurrence of dyspepsia, nausea and cutaneous edema were greater in the ibuprofen group than in the C. domestica group, there was no statistical difference. The single most prevalent effect in the C. domestica group was diarrhea, but with no statistical difference as well. In addition, two patients in the ibuprofen group had melena.[44]
The study by Gupte et al. (2019) also used ibuprofen as a comparator; however, they only described the occurrence of adverse events in the treatment group. Two patients reported heartburn and nausea after taking the drug, these patients dropped out of the study. One patient had a rash and itchiness over the entire body after two doses of drug ingestion that was attributed to an idiosyncratic effect, this patient was withdrawn from the study.[46]
In the study by Shep et al. (2019), the comparator was diclofenac. The occurrence of adverse events was significantly lower in the group receiving curcumin (13%) compared to the group receiving diclofenac (38%). The most common adverse events were nausea, diarrhea, abdominal pain / acidity and flatulence, all effects reported were mild and transient. The relative risk of nausea and diarrhea was reduced to 80 and 60% in the curcumin group, respectively.[45]
In studies that evaluated laboratory parameters, no significant changes were found in blood or urine that could be related to the occurrence of adverse events.[45,46]
Treatment adherence and rescue medication
Three amoung the four included studies described that adherence to the therapeutic regimen would be assessed by each patient during visits,[43-45] however in only two of them were the results reported. In one of the studies, adherence to the therapeutic regimen was better in the control group,[43] while there was no difference between adherence to the regimen between the two treatments in the study by Kuptniratsaikul et al. (2014).
In two studies, paracetamol / ranitidine,[45] and tramadol,[44] were used as rescue drugs. The need to use paracetamol as a rescue medication was greater in the curcumin group (21%) than in the diclofenac group (17%), however, with no statistical difference. Regarding ranitidine, none of the patients in the curcumin group needed to use the rescue drug, while 28% of patients in the diclofenac group needed to use ranitidine.[50] There was no difference in the use of tramadol as a rescue drug between the groups C. domestica (2.7%) and ibuprofen (1.1%).[44]
Treatment Evaluation
Three studies assessed patient satisfaction with treatments, there were no differences in patient satisfaction with treatment.[43-45] In the study by Shep et al. (2019), curcumin treatment was rated as excellent or good by 16% and 77% of patients, respectively.[45] In the study by Kuptniratsaikul et al. (2014), 64.3% of the patients in the C. domestica group considered that there was an improvement in the clinical picture, 33.9% were indifferent and 1.8% considered that there was a worsening, 97.1% demonstrated to be satisfied with the treatment.[44] In the study by Kuptniratsaikul et al. (2009), most patients indicated a level of satisfaction from moderate (46.7%) to high (44.4%) with treatment with C. domestica.
DISCUSSION
This scope review identified and methodologically assessed all available evidence as of the date of writing of the review (February 2020) related to the use of Curcuma longa or its derivatives curcuminoids / curcumin in the treatment of knee osteoarthritis compared to treatment with NSAIDs. In four studies found, only one study registered as a double blind randomized clinical trial, and three described as randomized controlled trials, of which only two listed in the clinical trial records.
This review did not include studies that compared Curcuma longa or its derivatives with placebo. The mechanism of action of curcumin is similar to the mechanism of action of NSAIDs. To avoid interference from synergistic effects in the assessment of outcomes, studies in which the use of Curcuma or its derivatives were associated with other drugs or extracts were not included from other medicinal plants so that the response rate for the treatment in question and the possible occurrence of adverse events could be assessed exclusively.
The main objective of treating osteoarthritis with analgesics and NSAIDs is pain relief and can cause serious adverse events in the gastrointestinal tract and cardiovascular system. Thus, the aim of this scope review was to evaluate the evidence related to the effects of use of Curcuma longa in the treatment of osteoarthritis and the possible benefits in relation to treatment with NSAIDs. Evidence from this scope review shows that treatment for 4 to 12 weeks with a dose of Curcuma extract that varied from 1500 to 2000 mg per day can reduce the symptoms of osteoarthritis in a similar way to treatment with diclofenac or ibuprofen, with less potential to cause adverse events. However, the methodological quality of the studies included in this review and the sample size does not allow definitive conclusions to be drawn.
Methodological flaws were evident in the conduct and reporting of all studies, none of the studies reported having adhered to the CONSORT guidelines (Consolidated Standards of Reporting Trials).[48] Although all studies carried out randomization, in all studies there were problems with sample size and dropouts. Regarding the knowledge of the treatment, one of the studies was of the open type and of the three that reported having performed some type of blinding, only in one study was the blinding of both patients and researchers described. In the allocation of patients, only one study did not report whether the allocation of treatments was blunted.
Osteoarthritis is an inflammatory disease, with the altered inflammatory state being the underlying cause of OA and mechanical stress the inducing cause. Patients with early arthroscopic manifestation of OA and knee pain, but with normal radiographic findings, exhibited immunohistological parameters related to inflammation in synovial tissue (tumor necrosis factor alpha (TNFα), interleukin 1 beta (IL-1β), nuclear factor κB ( NF-κB) and cyclooxygenase 2 (COX-2)) significantly higher when compared to patients with late osteoarthritis who required arthroplasty.[49] Oxidative stress is involved in the inflammatory process of osteoarthritis. Under the stimulation of TNFα and IL-1β there is a reduction in chondrocyte production by adenosine triphosphate due to the inhibition of complex I in the mitochondrial respiratory chain, thus reducing the potential of the mitochondrial membrane. At the same time, nitric oxide, oxygen-free species and superoxide anions released by macrophages and mitochondrial dysfunction can result in chondrocyte death.[48]
The first-line treatment for osteoarthritis is paracetamol, while oral and topical non-steroidal anti-inflammatory drugs (NSAIDs) are recommended as second-line treatment,[20] these treatments are aimed at relieving the symptoms of OA, but not modify the underlying cause of the disease, which is chronic inflammation. Non-selective NSAIDs are associated with gastrointestinal toxicity and selective NSAIDs are associated with acute myocardial infarction.[21,51]
Curcumin can suppress TNF-α production by macrophages, inhibit NF-κB activation,[52,53] and inhibit NF-κB translocation to the nucleus, preventing the inflammatory response of cells.[54] The negative regulation of NF-κB by curcumin plays an important role in suppressing the expression of TNF-α. Suppression of NF-kB is associated with inhibition of the expression of COX-2, NO, PGE2, IL-1β, IL-6, IL-8, MMP-3 and MMP-9 in human chondrocytes.[55,56] Curcumin also prevents decreased expression of Bcl-2 and Bcl-XL and increased expression of Bax and caspase-3 stimulated by IL -1β, reducing the inflammatory response.[54,57]
CONCLUSION
Although this scope review indicates that the oral administration of Curcuma reduces the symptoms of osteoarthritis, some limitations were found, such as the inclusion of only four randomized clinical trials, the small sample size (n = 42 to 367 participants), the evaluation of the outcomes in these studies have been performed using different measures (VAS, WOMAC, KOOS). It is noteworthy that, although different preparations of Curcuma longa were used in the included studies, the reduction in symptoms indicates that curcuminoids act by decreasing the inflammatory process of OA. These limitations indicate the need for randomized clinical trials of high methodological quality, following the guidelines of CONSORT (Consolidated Standards of Reporting Trials),[48] with a larger number of participants and a longer treatment period. It is indicated that the treatment with extracts of Curcuma longa or its derivatives should be carried out with extracts that have a known and standardized composition. The association of Curcuma longa with other plant extracts or drugs should be avoided in order to confirm the therapeutic efficacy of Curcuma longa in the treatment of osteoarthritis and its benefits over conventional treatments.
ACKNOWLEDGEMENT
This study was carried out through the Specialization Course in Health Technology Assessment funded by the Ministry of Health.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ABBREVIATIONS
AO: Osteoarthritis; RA: Rheumatoid Arthritis; NSAIDs: Nonsteroidal Anti-inflammatory Drugs; COX-2: Cyclooxygenase 2; VAS: Visual Analogue Scale; WOMAC: Western Ontario and McMaster Universities Arthritis Index; KOOS: Knee Injury and Osteoarthritis Outcome Score; CONSORT: Consolidated Standards of Reporting Trials; TNFα: Tumor Necrosis Factor alpha; IL-1β: Interleukin 1 beta; NF-Κb: Nuclear Factor κB.
REFERENCES
1. Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35. doi: 10.1002/art.23176, PMID 18163497.
2. Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford). 2005;44(1):7-16. doi: 10.1093/rheumatology/keh344, PMID 15292527.
3. Gupta S, Hawker GA, Laporte A, Croxford R, Coyte PC. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology (Oxford). 2005;44(12):1531-7. doi: 10.1093/rheumatology/kei049, PMID 16091394.
4. Ling SM, Patel DD, Garnero P, Zhan M, Vaduganathan M, Muller D, et al. Serum protein signatures detect early radiographic osteoarthritis. Osteoarthritis Cartilage. 2009;17(1):43-8. doi: 10.1016/j.joca.2008.05.004, PMID 18571442.
5. Hulejová H, Barešová V, Klézl Z, Polanská M, Adam M, Šenolt L. Increased level of cytokines and matrix metalloproteinases in osteoarthritic subchondral bone. Cytokine. 2007;38(3):151-6. doi: 10.1016/j.cyto.2007.06.001, PMID 17689092.
6. Martel-Pelletier J, Boileau C, Pelletier JP, Roughley PJ. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol. 2008;22(2):351-84. doi: 10.1016/j.berh.2008.02.001, PMID 18455690.
7. De Rezende MU, De Campos GC, Pailo AF. Current concepts in osteoarthritis. Acta Ortop Bras. 2013;21(2):120-2. doi: 10.1590/S1413-78522013000200010, PMID 24453655.
8. Coimbra IB, Pastor EH, Greve JMD, Puccinelli MLC, Fuller R, Cavalcanti Fde S, et al. Consenso brasileiro para o tratamento da osteoartrite (artrose). Rev Bras Reum. 2002.
9. Rejaili WA, Chueire AG, Cordeiro JA, Petean FC, de Carvalho Filho Gd. Avaliação do uso do Hylano GF-20 no pós-operatório de artroscopia de joelho por artrose. Acta Ortop Bras. 2005;13(1):20-3. doi: 10.1590/S1413-78522005000100005.
10. Braham R, Dawson B, Goodman C. The effect of glucosamine supplementation on people experiencing regular knee pain. Br J Sports Med. 2003;37(1):45-9; discussion 49. doi: 10.1136/bjsm.37.1.45, PMID 12547742.
11. Cote LG. Management of osteoarthritis. J Am Acad Nurse Pract. 2001;13(11):495-501. doi: 10.1111/j.1745-7599.2001.tb00014.x, PMID 11930514.
12. Belo JN, Berger MY, Reijman M, Koes BW, Bierma-Zeinstra SMA. Prognostic factors of progression of osteoarthritis of the knee: A systematic review of observational studies. Arthritis Rheum. 2007;57(1):13-26. doi: 10.1002/art.22475, PMID 17266080.
13. Natalio MA, Oliveira RBDC, Machado LVH. Osteoartrose: Uma revisão de literatura. Rev Digit Efedeportes. 2010.
14. Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheum Dis Clin North Am. 2013;39(1):1-19. doi: 10.1016/j.rdc.2012.10.004, PMID 23312408.
15. Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, et al. The global burden of hip and knee osteoarthritis: Estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(7):1323-30. doi: 10.1136/annrheumdis-2013-204763, PMID 24553908.
16. Stevens M, Paans N, Wagenmakers R, Van Beveren J, Van Raay JJAM, Van der Meer K, et al. The influence of overweight/obesity on patient-perceived physical functioning and health-related quality of life after primary total hip arthroplasty. Obes Surg. 2012;22(4):523-9. doi: 10.1007/s11695-011-0483-1, PMID 21800224.
17. Puig-Junoy J, Ruiz Zamora A. Socio-economic costs of osteoarthritis: A systematic review of cost-of-illness studies. Semin Arthritis Rheum. 2015;44(5):531-41. doi: 10.1016/j.semarthrit.2014.10.012, PMID 25511476.
18. José FF. Osteoartrite: Fisiopatologia e tratamento medicamentoso. J bras med. 2013.
19. Haq I, Murphy E, Dacre J. Osteoarthritis. Postgrad Med J. 2003;79(933):377-83. doi: 10.1136/pmj.79.933.377, PMID 12897215.
20. Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of non-pharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64(4):465-74. doi: 10.1002/acr.21596, PMID 22563589.
21. Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137-62. doi: 10.1016/j.joca.2007.12.013, PMID 18279766.
22. McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363-88. doi: 10.1016/j. joca.2014.01.003, PMID 24462672.
23. Machado GC, Maher CG, Ferreira PH, Pinheiro MB, Lin CWC, Day RO, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: Systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225. doi: 10.1136/bmj.h1225, PMID 25828856.
24. Da Costa BR, Reichenbach S, Keller N, Nartey L, Wandel S, Jüni P, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: A network meta-analysis. Lancet. 2017;390(10090):e21-33. doi: 10.1016/S0140-6736(17)31744-0, PMID 28699595.
25. Baltzer AWA, Moser C, Jansen SA, Krauspe R. Autologous conditioned serum (Orthokine) is an effective treatment for knee osteoarthritis. Osteoarthritis Cartilage. 2009;17(2):152-60. doi: 10.1016/j.joca.2008.06.014, PMID 18674932.
26. Moskowitz RW, Hooper M. State-of-the-art disease-modifying osteoarthritis drugs. Curr Rheumatol Rep. 2005;7(1):15-21. doi: 10.1007/s11926-005-0004-0, PMID 15760576.
27. Hunter DJ. Pharmacologic therapy for osteoarthritis--the era of disease modification. Nat Rev Rheumatol. 2011;7(1):13-22. doi: 10.1038/nrrheum.2010.178, PMID 21079644.
28. Basedow M, Runciman WB, March L, Esterman A. Australians with osteoarthritis; the use of and beliefs about complementary and alternative medicines. Complement Ther Clin Pract. 2014;20(4):237-42. doi: 10.1016/j. ctcp.2014.08.002, PMID 25194303.
29. Ramsey SD, Spencer AC, Topolski TD, Belza B, Patrick DL. Use of alternative therapies by older adults with osteoarthritis. Arthritis Rheum. 2001;45(3):222-7. doi: 10.1002/1529-0131(200106)45:3<222::AID-ART252>3.0.CO;2-N, PMID 11409661.
30. Ravindran PN, Babu KN, Sivaraman K. Turmeric: The genus Curcuma; 2013.
31. Ammon HPT, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57(1):1-7. doi: 10.1055/s-2006-960004, PMID 2062949.
32. Jayaprakasha GK, Jagan Mohan Rao L, Sakariah KK. Chemistry and biological activities of C. longa. Trends Food Sci Technol. 2005;16(12):533-48. doi: 10.1016/j.tifs.2005.08.006.
33. Mau J. Composition and antioxidant activity of the essential oil from Curcuma zedoaria. Food Chem. 2003;82(4):583-91. doi: 10.1016/S0308-8146(03)00014-1.
34. Lobo R, Prabhu KS, Shirwaikar A, Shirwaikar A. Curcuma zedoaria Rosc. (white turmeric): A review of its chemical, pharmacological and ethnomedicinal properties. (white turmeric): A review of its chemical, pharmacological and ethnomedicinal properties. J Pharm Pharmacol. 2009;61(1):13-21. doi: 10.1211/jpp/61.01.0003.
35. Itokawa H, Shi Q, Akiyama T, Morris-Natschke SL, Lee KH. Recent advances in the investigation of curcuminoids. Chin Med. 2008;3:11. doi: 10.1186/1749-8546-3-11, PMID 18798984.
36. Su HCF, Horvat R, Jilani G. Isolation, purification, and characterization of insect repellents from Curcuma longa L. J Agric Food Chem. 1982;30(2):290-2. doi: 10.1021/jf00110a018.
37. Dixit S, Awasthi P. Chemical composition of Curcuma longa leaves and rhizome oil from the plains of Northern India. J Young Pharmacists. 2009;1(4). doi: 10.4103/0975-1483.59319.
38. Essien EE, Newby JS, Walker TM, Setzer WN, Ekundayo O. Chemotaxonomic characterization and in-vitro antimicrobial and cytotoxic activities of the leaf essential oil of Curcuma longa Grown in Southern Nigeria. Medicines (Basel). 2015;2(4):340-9. doi: 10.3390/medicines2040340, PMID 28930216.
39. Srimal RC. Turmeric: A brief review of medicinal properties. Fitoterapia. 1997.
40. Arksey H, O’Malley L. Scoping studies: Towards a methodological framework. International Journal of Social Research Methodology. 2005;8(1):19-32. doi: 10.1080/1364557032000119616.
41. Peters MDJ, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141-6. doi: 10.1097/XEB.0000000000000050, PMID 26134548.
42. Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018;169(7):467-73. doi: 10.7326/M18-0850, PMID 30178033.
43. Kuptniratsaikul V, Thanakhumtorn S, Chinswangwatanakul P, Wattanamongkonsil L, Thamlikitkul V. Efficacy and safety of Curcuma domestica extracts in patients with knee osteoarthritis. J Altern Complement Med. 2009;15(8):891-7. doi: 10.1089/acm.2008.0186, PMID 19678780.
44. Kuptniratsaikul V, Dajpratham P, Taechaarpornkul W, Buntragulpoontawee M, Lukkanapichonchut P, Chootip C, et al. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: A multicenter study. Clin Interv Aging. 2014;9:451-8. doi: 10.2147/CIA.S58535, PMID 24672232.
45. Shep D, Khanwelkar C, Gade P, Karad S. Safety and efficacy of curcumin versus diclofenac in knee osteoarthritis: A randomized open-label parallel-arm study. Trials. 2019;20(1):214. doi: 10.1186/s13063-019-3327-2, PMID 30975196.
46. Gupte PA, Giramkar SA, Harke SM, Kulkarni SK, Deshmukh AP, Hingorani LL, et al. Evaluation of the efficacy and safety of capsule longvida® optimized curcumin (solid lipid curcumin particles) in knee osteoarthritis: A pilot clinical study. J Inflamm Res. 2019;12:145-52. doi: 10.2147/JIR.S205390, PMID 31239749.
47. The Joanna Briggs Institute. Checklist for systematic reviews and research syntheses. Joanna Briggs Institute; 2016.
48. Turner L, Shamseer L, Altman DG, Weeks L, Peters J, Kober T, et al. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database Syst Rev. 2012;11:MR000030. doi: 10.1002/14651858.MR000030. pub2, PMID 23152285.
49. Benito MJ, Veale DJ, FitzGerald O, Van Den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64(9):1263-7. doi: 10.1136/ard.2004.025270, PMID 15731292.
50. Blanco FJ, Rego I, Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat Rev Rheumatol. 2011;7(3):161-9. doi: 10.1038/nrrheum.2010.213, PMID 21200395.
51. Bally M, Dendukuri N, Rich B, Nadeau L, Helin-Salmivaara A, Garbe E, et al. Risk of acute myocardial infarction with NSAIDs in real world use: Bayesian meta-analysis of individual patient data. BMJ. 2017;357:j1909. doi: 10.1136/bmj. j1909, PMID 28487435.
52. Singh S, Aggarwal BB. Activation of Transcription Factor NF-κB is Suppressed by Curcumin (Diferuloylmethane). J Biol Chem. 1995;270(42):24995-5000. doi: 10.1074/jbc.270.42.24995.
53. Aggarwal BB, Gupta SC, Sung B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br J Pharmacol. 2013;169(8):1672-92. doi: 10.1111/bph.12131, PMID 23425071.
54. Buhrmann C, Mobasheri A, Busch F, Aldinger C, Stahlmann R, Montaseri A, et al. Curcumin modulates nuclear factor kappaB (NF-kappaB)-mediated inflammation in human tenocytes in vitro: Role of the phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem. 2011;286(32):28556-66. doi: 10.1074/jbc. M111.256180, PMID 21669872.
55. Shakibaei M, John T, Schulze-Tanzil G, Lehmann I, Mobasheri A. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclooxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem Pharmacol. 2007;73(9):1434-45. doi: 10.1016/j.bcp.2007.01.005, PMID 17291458.
56. Mathy-Hartert M, Jacquemond-Collet I, Priem F, Sanchez C, Lambert C, Henrotin Y. Curcumin inhibits pro-inflammatory mediators and metalloproteinase-3 production by chondrocytes. Inflamm Res. 2009;58(12):899-908. doi: 10.1007/s00011-009-0063-1, PMID 19579007.
57. Yan FX, Feng YM, Zhi CM, Wei LD, Yang X Yi, Sun J Yi, et al. Strategy to suppress oxidative damage-induced neurotoxicity in PC12 cells by curcumin: The role of ROS-mediated DNA damage and the MAPK and AKT pathways. Mol Neurobiol. 2016.
Cite this article: Neto JFA, Paixão JA, Vale AED, David JM, David JPDL. The use of Curcuma longa and its Derivatives in the Treatment of Osteorthritis: A Scoping Review. Pharmacog Rev. 2022;16(31):12-21.