|
Pharmacogn Rev. 2022;16(31):1-6 A multifaceted peer reviewed journal in the field of Pharmacognosy and Natural Products www.phcogrev.com | www.phcog.net |
Review Article |
Ethnobotany, Chemical Constitution and Biological Activities of Moquilea Genus: A Systematic Review
Fabián Vinicio Delgado-Rodríguez*, Marta Porras-Navarro, Natalia Villalobos-Gätjens, Jeniffer Sandí-Flores, Valeria Chaves-Hernández
Fabián Vinicio Delgado-Rodríguez*, Marta Porras-Navarro, Natalia Villalobos-Gätjens, Jeniffer Sandí-Flores, Valeria Chaves-Hernández
Instituto de Investigaciones Farmacéuticas (INIFAR), Pharmacy College, University of Costa Rica (11501-2060), San Pedro de Montes de Oca, San José, COSTA RICA.
Correspondence
Prof. Fabián Vinicio Delgado-Rodríguez,
Instituto de Investigaciones Farmacéuticas (INIFAR), Pharmacy College, University of Costa Rica (11501-2060), San Pedro de Montes de Oca, San José, COSTA RICA.
E-mail: [email protected]
History
• Submission Date: 28-08-2021;
• Review completed: 27-09-2021;
• Accepted Date: 14-11-2021.
DOI : 10.5530/phrev.2022.16.1
Article Available online
http://www.phcogrev.com/v16/i31
Copyright
© 2022 Phcog.Net. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International license.

ABSTRACT
Moquilea (Chrysobalanaceae) is a taxon recently separated from previous Licania genus. Several plants from Moquilea genus have been studied for the evaluation of their biological properties and chemical constitution analysis, especially those used as edible material and as a medicinal agent in several countries from tropical zones of America where the plants are endemic. Considering these facts, the present systematic review has the aim to summarize available information about ethnobotany, chemical components and biological effects investigated for Moquilea plants. For this purpose, eight data bases (Pubmed, ACS publications, EBSCOhost, ScienceDirect, SciELO, SpringerLink, Tayor and Francis Online and Wiley Online Library) were search and 2116 results corresponding to full-text articles and book chapters were screened using defined inclusion criteria. Finally, information from 45 documents were included in the review. According to compilated information, there are few species with available data related to their ethnobotanical applications. However, there is a wide variety of biological activities reported for extracts and isolated components obtained from Moquilea plants, including antioxidant, antimicrobial, cytotoxic and gastroprotective effects. Polyphenols and triterpenes are the major secondary metabolites reported among Moquilea plants.
Key works: Activities, Ethnobotany, Extracts, Metabolites, Moquilea.
Cite this article: Delgado-Rodríguez FV, Porras-Navarro M, Villalobos-Gätjens N, Sandí-Flores J, Chaves-Hernández V. Ethnobotany, Chemical Constitution and Biological Activities of Moquilea Genus: A Systematic Review. Pharmacog Rev. 2022;16(31):1-6.
INTRODUCTION
Plants from current Moquilea taxon were former members of Licania genus.[1] This recent accepted taxonomic classification of Licania based on genetic data established that Moquilea plants must be classified as a separate genus from Licania.[1-3] According to this division, Moquilea genus is integrated by 54 species of trees original from Neotropics and several of them have been studied for the characterization of their biological activities and secondary metabolites.[1-4]
Some Moquilea plants have reported ethnobotanical applications. For example, M. platypus have been used in Central America to treat stomach ache, diarrhea and snake bites.[5,6] The leaves and fruits of this tree are consumed as food in the regions where the plant is endemic.[7-10] In Brazil, M. tomentosa fruits are considered as edible and their seeds are source of vegetal oil.[11,12] This kind of information has inspired several investigations of the chemical characterization and biological activity of Moquilea plants.
Studied biological effects for Moquilea plants are varied, ranging from in vitro antioxidant activity analysis to the observation of the gastroprotective effect in vivo.[13,14] In the case of secondary metabolites characterization, polyphenols and triterpenes are the major groups of compounds studied among Moquilea plants.[4,13] Considering these backgrounds, the aim of the present review is to summarize the available information related to ethnobotanical applications, biological activities and secondary metabolites identified in Moquilea species using a systematic approach for the search of relevant literature in the topic.
METHODS
Moquilea is now considered as a separated genus derived from a previous taxon corresponding to Licania genus, this in accordance to the current taxonomic classification.[1-3] Therefore, most species from Moquilea genus retain Licania as the first term of their scientific name in literature due to previous taxonomic classification. Taking into count this fact, the search terms “Licania” and “Moquilea” were employed in the following data bases: Pubmed, ACS publications, EBSCOhost, ScienceDirect, SciELO, SpringerLink, Tayor and Francis Online and Wiley Online Library. A total number of 2116 results were found corresponding to full-text articles and book chapters. Then, the content of the found documents were screened and 2077 titles were excluded according to following criteria: content irrelevant to the scope of the review (n=1860), plants not included in Moquilea genus according to the current taxonomic definition of the genus[1] (n=185), repeated results among data bases (n=15), incomplete information (n=15) and not recognized species name (n=2). According to this, 39 articles and book chapters from consulted data bases were included in the review. Additionally, we added six known references important to the topic that are not included in the searched data bases. Finally, the review includes 45 references published between 1992 and 2021.
ETHNOBOTANICAL APPLICATIONS
There are few species from Moquilea genus that have reported ethnobotanical applications according to the information obtained from consulted data bases. The most reported specie is M. platypus. The fruits of this tree are used as food in Belize. In this country, the decoction made of M. platypus seeds combined with garlic skin is used to treat diarrhea, while seed powder is swallowed and applied as poultice to treat snake bites.[5,15] In Nicaragua, decoction and infusions made of bark, leaves and fruits of M. platypus are used to treat stomach ache and diarrhea.[6] In Mexico, the concoction made of leaves of M. platypus is eaten as an appetizer and the fruit is also eaten. Ground seeds are used in the same country to treat dysentery and diarrhea.[7-9,16] In Panama, fruits of M. platypus are used as fodder for livestock.[10] M. platypus fruits are considered as edible material in Colombia.[17] In Brazil, M. salzmannii and M. tomentosa fruits are consumed as food. In this country, M. tomentosa seeds are used as source of vegetal oil.[11,12]
BIOLOGICAL ACTIVITIES
There is a wide variety of biological activities studied for Moquilea plant extracts and metabolites. However, only four species have been studied. Most studies used in vitro approaches to measure their antioxidant potential, antimicrobial activity and cytotoxicity against tumoral cell lines among other effects. Moreover, there are studies using in vivo assays to observe the effect of plant extracts on gastrointestinal motility. The biological activities investigated for Moquilea plants are summarized in Table 1. Details about results of those studies are described below.
Table 1: Biological activities studied for Moquilea species.
| Species | Evaluated substance (plant section)* | Studied biological activities | References |
|---|---|---|---|
| M. kallunkiae | Methanol extract (St) | Antimalarial | [18,19] |
| M. platypus | Ethanol extract (L) | Gastroprotective | [14] |
| Aqueous extract (L) | Gastroprotective and gastrointestinal motility reduction | [20] | |
| Methanol extract (S) | Antibacterial | [21] | |
| M. pyrifolia | Methanol extract (L) | Molluscicidal and piscicidal | [22,23] |
| M. tomentosa | Ethanol extract (L) | Acaricidal | [24-27] |
| Ethanol extract (S) | Antioxidant | [28] | |
| Ethanol extract and fractions (S) | Antioxidant and cytotoxicity | [13] | |
| Ethanol extract (S) | Antioxidant and antibacterial | [29] | |
| Extract obtained by successive extraction with methanol 50 %, acetone 70 % and water (F) | Antioxidant | [12] | |
| Ethanol extract (L) | Antioxidant | [30] | |
| Aqueous saline extract: sodium chloride 0.85 % (S) | Antiviral and cytotoxicity | [31-33] | |
| Dichloromethane fraction from methanol extract (L) | Cytotoxicity | [34-36] | |
| Betulinic acid and oleanolic acid (F,L) | |||
| Mixture of triterpenic acids; 79 % betulinic, 13.1 % ursolic and 7.9 % tormentic (L) | |||
| Dichloromethane, ethanol, and ethyl acetate extracts (L) | Antileishmanial | [37] |
*F: fruits, L: leaves, S: seeds, St: stems.
Moquilea kallunkiae
Methanol extract from M. kallunkiae stems inhibited the growth of chloroquine-resistant Plasmodium falciparum strain (W2 Indochina) at concentration of 100 μg/mL with an inhibition percentage of 85.8 %.[18,19]
Moquilea platypus
The aqueous and ethanol extracts obtained from the leaves of M. platypus have demonstrated gastroprotective activity in models of gastric ulcer induction in rats by the administration of ethanol and indomethacin. The aqueous and ethanol extracts showed a protective index (PI) ranging from 80 % to 94 % against stomach ulcer formation induced with ethanol and indomethacin when they are oral administered at dose of 1000 mg/kg and 500 mg, respectively. Histological observation of stomach tissue isolated from control and treatment groups of those models showed that the extracts reduce tissue inflammation, necrosis, and leucocyte infiltration. Results obtained by pyloric ligation model of ulcer induction showed that the extracts promote the secretion of gastric mucus and, in the case of the aqueous extract, it reduces the acid and thiobarbituric acid reactive substances (TBARS) contents in gastric mucosa.[14,20] The aqueous extract does not affect intestinal motility but promotes a decrease in gastric empty according to red phenol model using rats as experimental animals.[20]
The methanol extract from M. platypus seeds has shown antibacterial activity against Staphylococcus aureus, Escherichia coli, Proteus mirabilis and Pseudomonas aeruginosa with average zones of inhibition in the range of 14 to 18 mm according to agar diffusion assay.[21]
Moquilea pyrifolia
Methanol extract from the leaves of M. pyrifolia has molluscicidal activity against the snails of Biomphalaria glabrata. The extract killed all the snails used in the test at concentration of 50 ppm, but it did not have piscicidal activity at concentration of 10 000 mg/L against Poecilia reticolata nor Carassius auratus.[22,23]
Moquilea tomentosa
Acaricidal activity has been reported for Moquilea tomentosa leaves against Rhipicephalus microplus larvae. Ethanol extract caused a mortality rate of 40,26 % of treated larvae at concentration of 600 mg/mL.[24-27]
Lima de Medeiros et al. reported results for the investigation of M. tomentosa fruits with the purpose of determine their nutritional and antioxidant potentials. According to this study, dry pulp has higher values for protein, fat, and dietary fiber (values up to 4.59 %, 4.06 % and 41.70 %, respectively) than dry seeds. However, dry seeds have more digestible carbohydrates (up to 69.01 %). Seeds also have higher contents of total tocopherols (1.76 mg/100 g). Ethanol extract from seeds of M. tomentosa has higher values for total phenolics (203 mg of gallic acid equivalents (GAE)/mg) and total flavonoids (29.88 mg of quercetin equivalents (QE)/mg) than pulp ethanol extract. Only the extract obtained from seeds has antioxidant activity with an inhibitory concentration (IC50) of 10.03 µg/mL on DPPH radical scavenging assay. The extract from seeds has shown IC50 values of 18 µg/mL without iron stress and 88 µg/mL with iron stress on thiobarbituric acid reactive substances (TBARS) assay. According to the ferric reducing antioxidant power (FRAP) assay, extracts from seeds have FRAP values up to 0.309 mM FeSO4/mg.[28]
The fractionation of an ethanol extract of M. tomentosa seeds with different solvents and the chemical analysis of the extract and their fractions indicate that ethyl acetate fraction had the highest values for total phenolics (201.83 mg GAE/g) and flavonoids (90.81 mg QE/g), but aqueous fraction had the highest tannins content (0.182 mg tannic acid equivalents/g). However, the evaluation of the antioxidant activity of the extract and its fractions shows that the methanol fraction was the most potent according to DPPH scavenging (IC50 of 26.30 µg/mL) and TBARS assays (IC50 of 105.72 and 8.55 µg/mL without iron stress and with iron stress, respectively). Ethyl acetate and methanol fractions have iron chelating activity with an iron complexation percentage near to 30 % at concentration of 1000 µg/mL. The ethanol extract has not shown cytotoxicity at test concentration of 250 µg/mL against human breast adenocarcinoma cell line (MCF-7) nor human colon adenocarcinoma cell line (Caco-2).[13]
Farias et al. reported the study of biological activities from another ethanol extract made of M. tomentosa seeds. According to this report, the extract has shown antioxidant activity under DPPH radical scavenging assay (IC50 of 216.72 μg/mL). The extract also had antibacterial activity against Staphylococcus aureus (minimal inhibitory concentration: 6.49 mg/mL) and inhibitory activity against acetylcholinesterase (inhibition percentage of 13.9 % at concentration 0.5 mg/mL).[29]
M. tomentosa fresh fruits have values for total phenolics, total anthocyanins and total carotenoids of 1236.42 mg of GAE/100 g, 2.96 mg of cyanidin 3-O-glucoside equivalents/100 g and 2.43 mg of β-carotenoids equivalents/100 g, respectively. The fruits have a total antioxidant activity of 14 271.69 µmol trolox equivalents/100 g according to DPPH scavenging activity assay.[12] The ethanol extract from M. tomentosa leaves also have antioxidant activity according to this assay (IC50 of 67.03 μg/mL).[30]
Extract of M. tomentosa seeds made using 0.85 % sodium chloride solution as extractant has shown a maximum non-toxic concentration on human larynx epidermoid tumor cell line (HEp-2) of 625 μg/mL and an IC50 of 7.66 mg/mL against cell viability. This extract inhibited the proliferation of acyclovir-resistant herpes simplex virus type 1 (ACVr-HSV1) on HEp-2 cultures with a IC50 value of 9 μg/mL giving a selective index of 851. The extract also can inactivate viral particles when they are set in direct contact before their addition to HEp-2 cultures (viral index of 2.46).[31-33]
Cytotoxicity evaluation of pentacyclic triterpenes isolated from M. tomentosa fruits and leaves indicates that betulinic acid and oleanolic acid are capable to inhibit the growth of human erythroleukemia cells lines, corresponding to K562 and multidrug resistant K562-Lucena 1 (K562/VCR). The compounds induce apoptosis and DNA fragmentation in exposed cell cultures. Similar results are reported for the dichloromethane fraction obtained from the methanolic extract of M. tomentosa leaves and the mixture of triterpenic acids (79% betulinic, 13.1%, ursolic and 7.9%, tormentic) obtained from it.[34-36]
Dichloromethane, ethanol, and ethyl acetate extracts from M. tomentosa leaves have not shown inhibitory activity against Leishmania amazonensis.[37]
CHEMICAL CONSTITUTION
Around ninety compounds have been detected or isolated from Moquilea plants. Polyphenols and triterpenes are the groups most frequently reported among studied species of the genus.
Polyphenols
The following compounds have been isolated from M. pyrifolia leaves using chloroform-methanol mixture 9:1 and methanol as extraction solvents: kaempferol (1), kaempferol-3-rhamnoside (2), kaempferol-3-arabinoside (3), kaempferol-3-(2’’-xylosil) rhamnoside (4), hypolaetin (5), 8-hydroxyeriodictyol (6), 8-hydroxynarigenin (7), myricetin (8), myricetin-3-rhamnoside (9), myricetin-3-(2’’-xylosyl)rhamnoside (10), quercetin (11), quercetin-3-rhamnoside (12), quercetin-3-arabinoside (13) and quercetin-3-(2’’-xylosyl)rhamnoside (14).[4,22,38,39]
Phytochemical screening of the ethanol extracts from M. tomentosa seeds and leaves indicates the presence of hydrolysable tannins, flavonols, flavononols, flavones, flavonones, catechins and saponins.[13,30] The extract was fractioned with solvents of different polarities for their analysis through high-performance liquid chromatography coupled to diode array detector (HPLC-DAD), these analyses demonstrate the presence of 1, 11, gallic acid (15), catechin (16), chlorogenic acid (17), caffeic acid (18), epicatechin (19), ellagic acid (20), kaempferol glycoside (21) and rutin (22) in the extract and all its fractions. Ethyl acetate fraction had the highest quantities of these compounds (ranging from 7.81 to 46.59 mg/g) except for methanol fraction that had the highest content of kaempferol (5.41 mg/g).[13] The analysis of the ethanol extract of M. tomentosa seeds using ultrahigh-performance liquid chromatography system coupled to mass spectrometry (UHPLC-MS) has shown the presence of the following components: gallocatechin (23), coumaric acid (24) and ferulic acid (25). Other compounds reported through the same analysis, which specific configuration and constitution must be defined in further research, are gallocatechin dimer isomer, naphthalenedicarboxylic acid hexoside, hydroxyjasmonic acid-O-hexoside, lariciresinol hexoside and quercetin pentosyl hexoside.[28]
M. platypus seeds have prodelphinidins and flavonoids according to UHPLC-MS analysis. This material has a total phenolics amount of 92 mg of GAE/g.[40] The structures of reported polyphenols in Moquilea plants are shown in Figure 1.
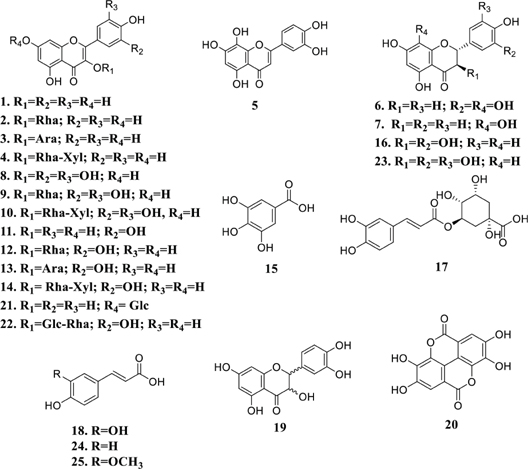
Figure 1: Polyphenols detected in Moquilea species.
Diterpenes and Triterpenes
Tinospinoside D (26) is a diterpene detected by UHPLC-MS analysis of the ethanol extract obtained from M. tomentosa seeds.[28] On the other hand, triterpenes corresponding to betulinic acid (27) and licanolide (28) are compounds isolated from hexane extract of M. tomentosa fruits. Oleanolic acid (29) has been obtained from methanol extract of the same fruits. Compounds corresponding to 27, lupeol (30), stigmasterol (31) and β-sitosterol (32) are substances isolated from hexane extract of M. tomentosa leaves, while 27, tormentic acid (33) and ursolic acid (34) are reported components of the methanol extract of the same plant material.[4,39,41,42] The ethyl acetate fraction from M. tomentosa fruits methanol extract contains 29. Dichloromethane fraction obtained from methanol extract of leaves of the same plant have 27, 33 and 34.[34]
The extraction of M. pyrifolia with chloroform and the further chromatographic fractionation of the extract have allowed the isolation of 27, 29, 30, 32, 34, α-amyrin (35), betulin (36) and uvaol (37). A similar procedure with the same plant material, using chloroform-methanol 9:1 mixture as extraction solvent, has yielded 33, 2α-hydroxy ursolic acid (38), maslinic acid (39), β-sitosterol-3-O-glucoside (40), 11α-hydroxybetulinic acid (41), 6β-hydroxybetulinic acid (42), 2α,3β-dihydroxyup-12-en-28-oic acid 3-(3′,4′-dihydroxybenzoyl ester) (43), 2α,3β,27-trihydroxylup-12-en-28-oic acid 3-(3′,4′-dihydroxybenzoyl ester) (44), ursolic acid 3-α-L-arabinoside (45), euscapic acid 28-β-D-glucopyranosyl ester (46), tormentic acid 28-β-D-glucopyranosyl ester (47), 2α,3α-dihydroxyurs-12-ene-28-oic acid (48) and euscapic acid (49).[22,39,43] Figure 2 summarizes the structure of diterpenes and triterpenes identified in Moquilea genus.
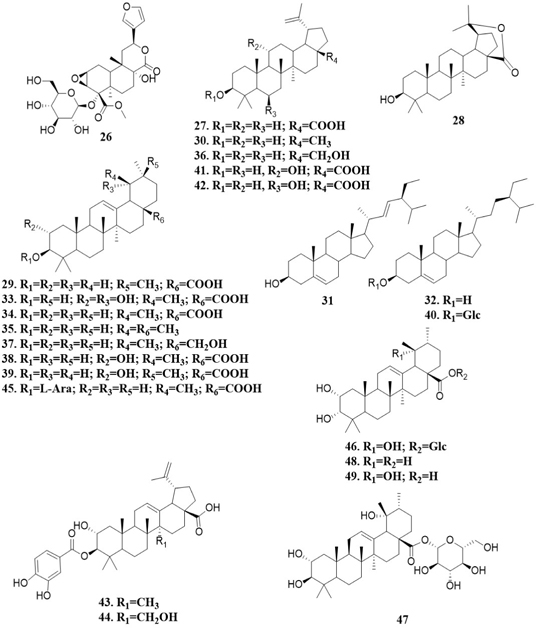
Figure 2: Terpenes isolated from Moquilea species.
Essential and Fixed Oils
The essential oil obtained from M. tomentosa fruits by hydrodistillation was analyzed by gas chromatography coupled to mass spectrometry (GC-MS). The results of this analysis indicate the presence of the following volatile compounds in the essential oil: hexanal (7.1-16.7 %) (50), ethyl butyrate (2.1-3.1%) (51), 3-hexene-1-ol (2.1-2.5%) (52), hexanol (11.1-33,5 %) (53), 3-hexanone-2-ol (21.0 %) (54), cyclopentyl ethanone (1.4 %) (55), butyl butyrate (3.4-6.4 %) (56), myrcene (2.1-6.4 %) (57), amyl butyrate (1.5-2.2 %) (58), terpinen-4-ol (0.2-1.0 %) (59), α-terpineol (0.8 %) (60), hexyl butyrate (1.0-3.5 %) (61), vinyl butyrate (2.3 %) (62), isopropyl butyrate (5.1 %) (63), trans-2-hexenal (3.7 %) (64), isoamyl acetate (0.6 %) (65), 4-heptanol (10.5 %) (66), nonane (1.8 %) (67), benzaldehyde (1.0 %) (68), sabinene (0.7 %) (69), 5-methyl-5-hexen-2-one (0.4 %) (70), ethyl hexanoate (1.4 %) (71), hexyl acetate (1.0 %) (72), pentyl isobutyrate (7.4 %) (73), isopentyl butyrate (5.4 %) (74), 1-octanol (0.4 %) (75), 1,2-heptanediol (3.6 %) (76), undecane (0.5 %) (77), cis-β-dihydro terpineol (0.1 %) (78), dodecane (0.2 %) (79), pentyl hexanoate (1.0 %) (80), safrol (1.6 %) (81), 2,4-decadienal (0.3 %) (82), hexyl hexanoate (0.2 %) (83), hexadecane (0.1 %) (84) and palmitic acid (0.6 %) (85).[44,45] Compounds 85 and palmitoleic acid (86) are fatty acids reported in hexane extracts obtained from M. tomentosa leaves and fruits, respectively.[41] Additionally, M. tomentosa seeds are known source of vegetal oil with a yield above 30 %.[46] Figure 3 shows the structure of volatile compounds and fatty acids reported in Moquilea species.
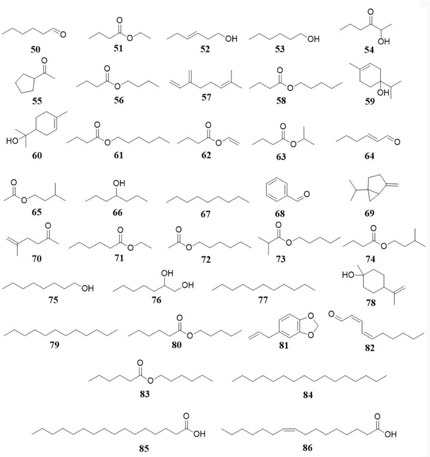
Figure 3: Volatile compounds and fatty acids present in Moquilea species.
CONCLUSION
Few species of the Moquilea genus have been studied, however, promissory results have been obtained from plants investigated, this suggest that further research of more plants of the genus could allow to find new bioactive extracts and compounds. In this sense, it is important to notice that the found information shows that most of the studied bioactive extracts do not have a chemical characterization of their compounds, and more important, in most cases the isolation and identification of major bioactive compounds responsible of the observed effects are pending tasks. Polyphenols, triterpenes and volatile compounds have been detected or isolated, but only few of them have been studied to examine their individual biological properties. Toxicological studies of bioactive extracts and metabolites are also necessary to have a better panorama of their applicability. In summary, Moquilea genus is a promising source of bioactive extracts and compounds, thus, further research, especially of less studied species, will help to get a better understanding in this topic.
ACKNOWLEDGEMENT
The authors express their gratitude to Instituto de Investigaciones Farmacéuticas (INIFAR) for supporting the research project that made possible this publication.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ABBREVIATIONS
ACVr-HSV1: Acyclovir-resistant herpes simplex virus type 1; Caco-2: Human colon adenocarcinoma cell line; DPPH: 2,2-diphenyl-1-picrylhydrazyl; FRAP: Ferric reducing antioxidant power; GAE: Gallic acid equivalents; HEp-2: human larynx epidermoid tumor cell line; HPLC-DAD: High-performance liquid chromatography coupled to diode array detector; IC50: Half-maximal inhibitory concentration; K562/VCR: Human erythroleukemia cells line; MCF-7: Human breast adenocarcinoma cell line; PI: Protective index; QE: Quercetin equivalents; TBARS: Thiobarbituric acid reactive substances; UHPLC-MS: Ultrahigh-performance liquid chromatography coupled to mass spectrometry.
REFERENCES
1. Sothers CA, Prance GT, Chase MW. Towards a monophyletic Licania: a new generic classification of the polyphyletic Neotropical genus Licania (Chrysobalanaceae). Kew Bull. 2016;71(4):58. doi: 10.1007/S12225-016-9664-3.
2. International Plant Name Index (IPNI) [homepage on the Internet]. The Royal Botanic Gardens, Kew, Harvard University Herbaria and libraries and Australian National Botanic Gardens; c2021; updated 2021 Apr 9; [cited Apr 18 2021]. Available from: https://www.ipni.org/.
3. Plants of the World Online [homepage on the Internet]. Royal Botanic Gardens, Kew; c2019; updated 2021; [cited Apr 18 2021]. Available from: http://www.plantsoftheworldonline.org/.
4. Feitosa EA, Xavier HS, Randau KP. Chrysobalanaceae: traditional uses, phytochemistry and pharmacology. Rev Bras Farmacogn. 2012 Oct;22(5):1181-6. doi: 10.1590/S0102-695X2012005000080.
5. Balick MJ, Arvigo R. Messages from the Gods A guide for Useful Plants of Belize. Oxford: Oxford University Press, The New York Botanical Garden; 2015. p. 254-5.
6. Barrett B. Medicinal plants of Nicaragua’s Atlantic Coast. Econ Bot. 1994;48(1):8-20. doi: 10.1007/BF02901375.
7. Zamora-Martínez MC, de Pascual Pola CN. Medicinal plants used in some rural populations of Oaxaca, Puebla and Veracruz, Mexico. J Ethnopharmacol. 1992 Jan;35(3):229-57. doi: 10.1016/0378-8741(92)90021-i, PMID 1548896.
8. Segura S, Fresnedo J, Mathuriau C, López J, Andrés J, Muratalla A. The edible fruit species in Mexico. Genet Resour Crop Evol. 2018;65(6):1767-93. doi: 10.1007/s10722-018-0652-3.
9. Mapes C, Basurto F. Biodiversity and edible plants of Mexico. In: Lira R, Casas A, Blancas J, editors. Ethnobotany of Mexico. Ethnobiology. New York: Springer; 2016. p. 83-131.
10. Love B, Spaner D. A survey of small-scale farmers using trees in pastures in Herrera Province, Panama. J Sustain For. 2005;20(3):37-65. doi: 10.1300/J091v20n03_03.
11. Teixeira N, Melo JCS, Batista LF, Paula-Souza J, Fronza P, Brandão MGL. Edible fruits from Brazilian biodiversity: a review on their sensorial characteristics versus bioactivity as tool to select research. Food Res Int. 2019 May;119:325-48. doi: 10.1016/j.foodres.2019.01.058, PMID 30884663.
12. Moreira-Araújo RSdR, Barros NVdA, Porto RGCL, Brandão AdCAS, Lima Ad, Fett R Amorim Serpa Brandão A, de Lima A, et al. Bioactive compounds and antioxidant activity three fruit species from the Brazilian Cerrado. Rev Bras Frutic. 2019;41(3):e-11. doi: 10.1590/0100-29452019011.
13. Parra Pessoa I, Lopes Neto JJ, Silva de Almeida T, Felipe Farias D, Vieira LR, Lima de Medeiros J, Augusti Boligon A, Peijnenburg A, Castelar I, Fontenele Urano Carvalho A. Polyphenol composition, antioxidant activity and cytotoxicity of seeds from two underexploited wild Licania species: L. Rigida and L. tomentosa. Molecules. 2016 Dec 21;21(12):1755. doi: 10.3390/molecules21121755, PMID 28009846.
14. Orozco Aguilar J, Chavarría Rojas M, Alvarado Barboza G, Cordero García E, Morales Acuña JA, Retana Salazar A, González Camacho S. Actividad gastroprotectora del extracto etanólico de hojas de <i>Licania platypus</i> (Hemsl.) Fritish Rev Med UCR. 2017;11(1):1. doi: 10.15517/rmucr.v11i1.30430.
15. Giovannini P, Howes MR. Medicinal plants used to treat snakebite in Central America: review and assessment of scientific evidence. J Ethnopharmacol. 2017 Mar 6;199:240-56. doi: 10.1016/j.jep.2017.02.011, PMID 28179114.
16. Ochoterena-Booth H. Tzontzápotl: licania platypus (Chrysobalanaceae), un recurso de potencial económico empleado desde el México prehispánico. Bot Sci;(52). doi: 10.17129/botsci.1408.
17. López Diago D, García Castro NJ. Frutos silvestres comestibles de Colombia: diversidad y perspectivas de uso. Biota;22(2):16-55. doi: 10.21068/c2021. v22n02a02.
18. Calderón AI, Simithy-Williams J, Gupta MP. Antimalarial natural products drug discovery in Panama. Pharm Biol. 2012 Jan;50(1):61-71. doi: 10.3109/13880209.2011.602417, PMID 22196582.
19. Munigunti R, Nelson N, Mulabagal V, Gupta MP, Brun R, Calderón AI. Identification of oleamide in Guatteria recurvisepala by LC/MS-based Plasmodium falciparum thioredoxin reductase ligand binding method. Planta Med. 2011 Oct;77(15):1749-53. doi: 10.1055/s-0030-1271080, PMID 21567357.
20. Orozco Aguilar J, Chavarría Rojas M, Sandí Flores J, Alvarado Barboza G, Cordero García E, Morales Acuña JA, Retana Salazar A, González Camacho S. Efecto del extracto acuoso de hojas de <i>Licania platypus (Hemsl.) Fritsh</i>. Sobre la protección gástrica y la motilidad gastrointestinal. Rev Med UCR. 2017;11(2):2. doi: 10.15517/rmucr.v11i2.34575.
21. Smith RA, Calviello CM, Dermarderosian A, Palmer ME. Evaluation of antibacterial activity of Belizean plants: an improved method. Pharm Biol. 2000;38(1):25-9. doi: 10.1076/1388-0209(200001)3811-BFT025, PMID 21214435.
22. Braca A, Bilia AR, Mendez J, Pizza C, Morelli I, De Tommasi N. Chemical and biological studies on Licania Genus. Stud Nat Prod Chem. 2003;28(I):35-67. doi: 10.1016/S1572-5995(03)80138-2.
23. Bilia AR, Braca A, Mendez J, Morelli I. Molluscicidal and piscicidal activities of Venezuelan Chrysobalanaceae plants. Life Sci. 2000;66(4):PL53-9. doi: 10.1016/s0024-3205(99)00600-1, PMID 10665990.
24. Silva JJMD, Campanharo SC, Paschoal JAR. Ethnoveterinary for food-producing animals and related food safety issues: A comprehensive overview about terpenes. Compr Rev Food Sci Food Saf. 2021 Jan;20(1):48-90. doi: 10.1111/1541-4337.12673, PMID 33443807.
25. Valente PP, Amorim JM, Castilho RO, Leite RC, Ribeiro MF. In vitro acaricidal efficacy of plant extracts from Brazilian flora and isolated substances against Rhipicephalus microplus (Acari: Ixodidae). Parasitol Res. 2014 Jan;113(1):417-23. doi: 10.1007/s00436-013-3670-2, PMID 24221889.
26. Adenubi OT, Fasina FO, McGaw LJ, Eloff JN, Naidoo V. Plant extracts to control ticks of veterinary and medical importance: a review. S Afr J Bot. 2016 Apr;105:178-93. doi: 10.1016/j.sajb.2016.03.010.
27. Adenubi OT, Ahmed AS, Fasina FO, Mc Gaw. J, Eloff JN, Naidoo V. Pesticidal plants as a possible alternative to synthetic acaricides in tick control: A systematic review and meta-analysis. Ind Crops Prod. 2018 Nov;123(1):779-806.
28. Lima de Medeiros J, Silva de Almeida T, Joaquim Lopes Neto J, Carlos Pereira Almeida Filho LC, Riceli Vasconcelos Ribeiro P, Sousa Brito E, Antonio Morgano M, Gomes da Silva M, Felipe Farias D, Fontenele Urano Carvalho A. Chemical composition, nutritional properties, and antioxidant activity of Licania tomentosa (Benth.) fruit. Food Chem. 2020 May 30;313:126117. doi: 10.1016/j.foodchem. 2019.126117.
29. Farias DF, Souza TM, Viana MP, Soares BM, Cunha AP, Vasconcelos IM, Ricardo NM, Ferreira PM, Melo VM, Carvalho AF. Antibacterial, antioxidant, and anticholinesterase activities of plant seed extracts from Brazilian semiarid region. BioMed Res Int. 2013;2013:510736. doi: 10.1155/2013/510736.
30. Silva JAd, Martins JdS, Paulino MLVdB, Almeida ASd, Pavão JMdSJ, Santos AFd. Prospecção fitoquímica e determinação do potencial antioxidante in vitro da Licania tomentosa (Benth.). Div Journ. 2021;6(2):2099-108. doi: 10.17648/diversitas-journal-v6i2-908.
31. Miranda MM, Gonçalves JL, Romanos MT, Silva FP, Pinto L, Silva MH, Ejzemberg R, Granja LF, Wigg MD. Anti-herpes simplex virus effect of a seed extract from the tropical plant Licania tomentosa (Benth.) Fritsch (Chrysobalanaceae). Phytomedicine. 2002 Oct;9(7):641-5. doi: 10.1078/094471102321616463, PMID 12487329.
32. Thompson KD. Herbal extracts and compounds active against herpes simplex virus. In: Hassan Khan MT, Ather A, editors. Advances in phytomedicine. Vol. 2. Elsevier Science; 2006. p. 65-86.
33. Garber A, Barnard L, Pickrell C. Review of Whole Plant Extracts With Activity Against Herpes Simplex Viruses In Vitro and In Vivo. J Evid Based Integr Med. 2021;26:2515690X20978394. doi: 10.1177/2515690X20978394. PMID 33593082.
34. Fernandes J, Castilho RO, da Costa MR, Wagner-Souza K, Coelho Kaplan MA, Gattass CR. Pentacyclic triterpenes from Chrysobalanaceae species: cytotoxicity on multidrug resistant and sensitive leukemia cell lines. Cancer Lett. 2003 Feb 20;190(2):165-9. doi: 10.1016/s0304-3835(02)00593-1, PMID 12565171.
35. Bernardes de Andrade Carli C, Bassi Quilles M, Zeppone Carlos I. Natural products with activity against multidrug-resistant tumor cells. In: Kumar Rhai M, Volodymyrivna Kon K, editors. Fighting multidrug resistance with herbal extracts, essential oils and their components. United States: Academic Press, Elsevier; 2013. p. 237-44.
36. Senthilkumar R, Chen BA, Cai XH, Fu R. Anticancer and multidrug-resistance reversing potential of traditional medicinal plants and their bioactive compounds in leukemia cell lines. Chin J Nat Med. 2014 Dec;12(12):881-94. doi: 10.1016/S1875-5364(14)60131-X, PMID 25556059.
37. Ribeiro TG, Chávez-Fumagalli MA, Valadares DG, Franca JR, Lage PS, Duarte MC, Andrade PH, Martins VT, Costa LE, Arruda AL, Faraco AA, Coelho EA, Castilho RO. Antileishmanial activity and cytotoxicity of Brazilian plants. Exp Parasitol. 2014 Aug;143:60-8. doi: 10.1016/j.exppara.2014.05.004, PMID 24846006.
38. Bilia AR, Ciampi L, Mendez J, Morelli I. Phytochemical investigations of Licania genus. Flavonoids from Licania pyrifolia. Pharm Acta Helv. 1996 Aug;71(3):199-204. doi: 10.1016/0031-6865(96)00009-X.
39. Carnevale Neto F, Pilon AC, da Silva Bolzani V, Castro-Gamboa I. Chrysobalanaceae: secondary metabolites, ethnopharmacology and pharmacological potential. Phytochem Rev. 2013;12(1):121-46. doi: 10.1007/s11101-012-9259-z.
40. Kim J, Gripenberg S, Karonen M, Salminen JP. Seed tannin composition of tropical plants. Phytochemistry. 2021;187:112750. doi: 10.1016/j.phytochem.2021.112750.
41. Castilho Oiliveira R, Kaplan Coelho MA. Chemical constituents of Licania tomentosa Benth. (Chrysobalanaceae). Quim Nova. 2008;31(1):66-9.
42. Castilho RO, de Oliveira RR, Kaplan MA. Licanolide, a new triterpene lactone from Licania tomentosa. Fitoterapia. 2005 Sep;76(6):562-6. doi: 10.1016/j.fitote. 2005.04.018, PMID 15970398.
43. Bilia AR, Morelli I, Mendez J. New Lupane derivatives from the leaves of Licania pyrifolia. J Nat Prod. 1996;59(3):297-300. doi: 10.1021/np960134j.
44. Castilho RO, Kaplan MAC. Volatile components of Oiti fruit (Licania tomentosa Benth.). Rec Nat Prod. 2010;4(4):238-41.
45. Andrade EHA, Zoghbi MdGB, Maia JGS. Constituintes voláteis dos frutos de Licania tomentosa Benth Acta Amaz. 1998;28(1):55-. doi: 10.1590/1809-43921998281058.
46. Harand W, Pinho RS, Felix LP. Alternative oilseeds for Northeastern Brazil: unrevealed potential of Brazilian biodiversity. Braz J Bot. 2016;39(1):169-83. doi: 10.1007/s40415-015-0233-z.
Cite this article: Delgado-Rodríguez FV, Porras-Navarro M, Villalobos-Gätjens N, Sandí-Flores J, Chaves-Hernández V. Ethnobotany, Chemical Constitution and Biological Activities of Moquilea Genus: A Systematic Review. Pharmacog Rev. 2022;16(31):1-6.